Research Article, J Nucl Ene Sci Power Generat Technol Vol: 9 Issue: 2
Selective Removal of Cs+ and Sr2+ in Seawater by Novel Zeolite Honeycomb Modeling
Md. Shakilur Rahman1,2*, Hitoshi Mimura2, Minoru Matsukura3 and Fatema Binte Amin4
1Nuclear Safety, Security and Safeguards Division, Bangladesh Atomic Energy Commission, Dhaka, Bangladesh
2Department of Quantum Science and Energy Engineering, Tohoku University, Aramaki-Aza-Aoba, Sendai, Japan
3Union Showa KK, Kounan Minato-ku, Tokyo, Japan
4Department of Chemistry, Jahangirnagar University, Savar, Dhaka
*Corresponding Author : Md. Shakilur Rahman, Nuclear Safety
Security and Safeguards Division
Bangladesh Atomic Energy Commission
Dhaka, Bangladesh
E-mail: shakilurssdl@baec.gov.bd
Received: August 12, 2019; Accepted: April 24, 2020; Published: April 30, 2020
Citation: Rahman MdS, Mimura H, Matsukura M, Amin FB (2020) Selective Removal of Cs+ and Sr2+ in Seawater by Novel Zeolite Honeycomb Modeling. J Nucl Ene Sci Power Generat Technol 9:2. doi: 10.4172/2325-9809.1000192
Abstract
The sorption removal of cesium and strontium ions from chemically synthesized fibrous mordenite (M) and zeolite type ‘A’ in the form of honeycomb in seawater consisting of competing cations Na+, Mg2+, Ca2+, and K+ have been investigated. The batch experiments were carried out for the effect of various parameters such as initial contact time, uptake with volume mass ratio, distribution coefficients (Kd) factor, and effect on temperature. The uptake (%) of Cs+ ions for mordenite honeycomb at V/m=100 cm3/g was measured to be 86.8% and 57.9% within 18 h in pure water and 15 h in seawater media, respectively. On the other hand, the uptake (%) of Sr2+ ions for type ‘A’ zeolite honeycomb was 99.2% within 24 h and 69.0% within 3 h in pure water and seawater media, respectively. The adsorption of Cs+ and Sr2+ by compact column on mordenite and type ‘A’ zeolite honeycomb at a flow rate of 1 cm3/min, and 3 cm3/ min were observed and found increasing with circulating time and flow rate that reaches maximum 64.5%, and 70.3% for Cs and 52.7% and 71.2% for Sr after 7 d, respectively. The adsorption increases with increasing flow rate indicates that the overall kinetics was dominated by external mass transfer with time of the sorption process in the column which is a favorable indicator. A mathematical relationship of adsorption with respect to time and mass volume ratio has been established. The solidification of zeolite shows an excellent immobilization of less than 1% at 1200ºC for both the zeolite has been obtained.
Keywords: Liquid radioactive waste; Cs+; Sr2+; Seawater; Cations; Uptake;
Power reactor;
PACS: 28.41.Kw; 28.41.Te
Introduction
The radionuclides of long half-life such as Cs and Sr are produced with high yields in the nuclear fission process which is considered to be the most hazardous in the nuclear waste effluents for the environment. To ensure the protection of human health and the environment in separating radioactive material from liquid water is still a challenge to develop highly selective removal technology of radionuclide of interest. 137Cs and 90Sr is a major contributor (0.3 percent) in activity and heat load produced by the fission in a nuclear reactor. Moreover, 137Cs and 90Sr are strong beta emitter with long half-life of 30 and 29.7 years respectively and hence it is the source of Cerenkov radiation which resulting a challenge of shielding problem. Selective removal of cesium and strontium is a critical issue for the treatment of liquid radioactive waste generated from nuclear power plant. The treatment of aqueous radioactive solutions requires the concentration of dissolved metal ions followed by the recovery or secure disposal [1].
Among the various processes or techniques for the treatment of aqueous contaminated liquid solutions, ion exchange technology is considered to be an attractive process for the treatment of contaminated water or liquid waste water because of its high efficiency, selectivity and simplicity. Hence, the selection of the ion exchange material is an important task. In past a wide range of materials containing different chemical and physical properties either naturally or synthetic are used for this technique. Inorganic ion exchanger materials emerged increasingly important replacement for conventional ion exchange resin for the decontamination of radioactive water generated from nuclear facilities. Several inorganic ion exchangers such as zeolite, hexaferrocyanites (CsTreat, SrTreat, CoTreat), silicotitanates, are presently being used.
Ion exchange properties of zeolite have received great attention especially for the applications of radioactive liquid water treatment [2-9]. This inorganic material possesses high exchange capacity, element selectivity, as well as good resistance to radiation and heat with advantages with respect to immobilizations and final disposal in compared to the organic ion exchanger [10-14]. It has high ion exchange capacity and a particular affinity for heavy metal cations hence the adsorption of 137Cs and 90Sr for the liquid solution and holds them in its three-dimensional crystal frame work [12]. The aluminosilicate zeolite act as efficient porous exchange media and its cations exchange properties are widely used in nuclear industry, detergent industry, and environmental protection [15-18]. The potential applications of zeolite and selective ion exchangers have been documented by Dyer [19].
Several works of sorption studies [7-9,12,20-22] of zeolite have been attempt in past for liquid radioactive waste treatment. The recent studies were conducted by A.M. El-Kamash et al. [8] where synthesized and evaluated zeolite type ‘A’ was used for the removal of Cs and Sr ions from aqueous solution in batch and fixed bed column operation. The total metal ion uptake and overall bed capacity decreased by increasing flow rate. But in the present study, mordenite and zeolite type ‘A’ in the form of fabulous honeycomb have been used for the separation of Cs+ and Sr2+ in seawater (containing major competing cation Na+~10,500 ppm, Mg2+~1,350 ppm, Ca2+~400 ppm and K+~380 ppm), where vertical column was used. The uptake increases with increasing flow rate that permitting higher flow rate for decontamination.
On the other hand, the development of stable solidification is an important subject for the safety denomination of radioactive decontaminated water. Cesium and strontium forms of zeolites recrystallize easily to stable aluminum silicate minerals [23,24] and cesium and strontium feldspar CsAlSi3O8 and SrAl2Si2O8 in these minerals have been noted as suitable phases for fixation of Cs and Sr on the basis of their refectory nature and leach resistance [25].
The present works deals with the measurement of efficiency of mordenite (M) and ‘A’ type zeolite in the form of honeycomb as a cation exchanger for the selective removal of cesium and strontium ions in seawater. The present study also deals with effects of temperature and immobilization ability of Cs and Sr i.e. trapping and self-sintering abilities. The experiment is designed on the basis of Fukushima Daiichi NPP accident due to the Great East Japan Earthquake where a significant amount of high level liquid radioactive waste generated and spilled over in a confined zone in the Pacific Ocean from the facilities. Hence, the development of highly selective ion exchange materials will give a new possibility for the separation of Cs and Sr from liquid radioactive waste solution even at very high concentration of inactive salts. This can give a good economic solution as well as environmental remediation.
Experimental Details
Materials and methods
Zeolite: Zeolites are crystalline microporous aluminosilicates that are build-up of three dimensional framework [SiO4]4- and [AlO4]5- tetrahydar lined by sharing oxygen atom and weakly bounded cations. It is a volcanic mineral with unique characteristics which comprises of hydrogen, oxygen, silicon and aluminum and arrange in an interconnected lattice structure. The elemental arrangement of the natural zeolite with gives rise in the frame of honeycomb. The diameter of the connecting channel varies from 2.5 Å to 5 Å. Zeolite has a high ion-exchange capacity and particularly affinity to metallic ion give rise the use for the separation of 137Cs and 90Sr from contaminated liquid in nuclear technology. In the present study, Mordenite and zeolite type ‘A’ are used in the form of honeycomb skeleton. The zeolites honeycomb used in this study are supplied by Seibu Giken Co., Ltd. Japan. The elemental distributions of the zeolites are analyzed by Scanning Electron Microscope (SEM) and Energy-Dispersive x-ray Spectroscopy (EDS). The properties of zeolite honeycomb are given in Table 1. The XRD measurement was done with X-ray diffraction using Cu Kα radiation is shown in Figure 1.
Table 1: The main properties of the zeolite honeycomb.
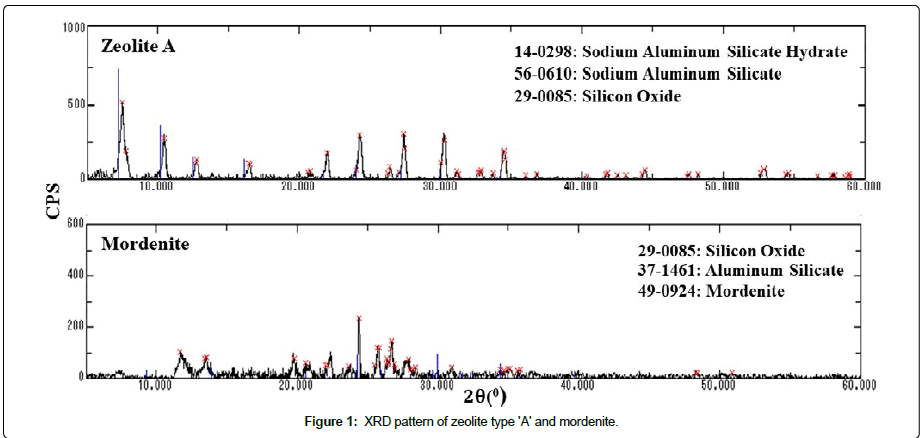
Figure 1: XRD pattern of zeolite type 'A' and mordenite.
Chemicals and reagents: All reagents used in the present study were of AR grade chemical which were used without any further purification. Cesium and strontium ions are supplied as standard solution 1000 ppm from CsCl and SrCl2.6H2O that dissolved in distilled water. Stock solution 0.5 M Cs and 0.5 M Sr ion were prepared from CsCl and SrCl2.6H2O dissolved in seawater. Seawater is collected from Matsushima bay and filtered to be used.
Batch adsorption studies: The adsorption efficiency of the zeolites is carried out by batch technique to obtain equilibrium conditions. The experiments were carried out in a fixed pH range at laboratory temperature (25°C). In order to observe the absorption capacity of the zeolites, the honeycomb zeolites are meshed with a size of 8-32 mesh and 32-48 mesh. A 10 ppm Cs+ and Sr2+ ionic solutions are prepared from 1000 ppm standard solution in seawater and pure water media. A series of 20 ml test tube each containing 100 mg of zeolite and 10 ml solution of specific mesh size was used. The pH value of the whole experiment lies within 6.94-7.79. The test tubes were shaken by shaker maintain fixed temperature for 24 h to attain equilibrium. The concentration of Cs and Sr were measured by atomic absorption spectroscopy and inductively coupled plasma mass spectrometry system respectively.
The percentages of uptake of Cs and Sr ion from aqueous solution was computed from the equation (1)

Where, Ci and Cf referred to initial concentration of ion in aqueous phase, R represents the uptake or removal percentages.
The amount of adsorption at any time t, qt (mg/g) can be determined by the equation [26],

V represents volume of the ionic solution in litre (L), amd m be the weight of the solid used in mg.
Decontamination factor: The determination of several parameters such as ion exchange capacity, distribution coefficients, and decontamination factors are highly required for any ion exchange material. Moreover selectivity and stable conditions of the sorbent material are also important. Decontamination factor is very important that describe the ratio of contamination level before treatment to that after treatment. This factor is very useful for the selection of suitable material as sorbent and commonly used in radioactive waste management applications [27]. The amount of media required and the rate of exchange can be determined by the following equation:

Where DF represents the decontamination factor, V is volume of the liquid to be purified in ml, m be the mass of the ion exchange materials in gram, and Kd is the distribution coefficients that can be determined by the following equation:

Hence, the removal percentages (%) and Kd (ml/g) can be express by the following;

Column experiment: Highly selective ion exchangers can be utilized in many different ways. Most efficient way is to use columns with volume of 2-12 liters. Hence, used ion exchange columns are very easy to dispose in a small volume by solidification method. Small volume of ion exchanger in a compact system will give remarkable savings in treatment and disposal costs.
Column uptake studies were conducted to evaluate the column performance for Cs+ and Sr2+ removal by zeolites. The experiments were carried out with a vertical glass column (50 mmφ × 100 mmL) that packed with mordenite honeycomb and ‘A’ type zeolite of the same size. The solution of seawater (2,000 cm3, Matsushima bay) containing 10 ppm Cs+ or 10 ppm Sr2+ ions was circulated through column adsorption experiments were carried out using circulating methods with a at flow rate of 1 cm3/min and 3 cm3/min. The samples were putted into the column carefully so that no air remained between the particles, which helped attain compact layers. At the exit of the column, flow rate was controlled so as to get steady state conditions in the column. Sampling effluents was at the predetermined time intervals in order to investigate breakthrough point.
Results and Discussion
Uptake of Cs+ and Sr2+ in pure water and sea water media
Sorption experiments of mordenite and zeolite type ‘A’ were carried out with a fixed adsorbent dosage of 100 mg in 10 ppm Sr and Cs solution in order to achieve V/m=100. The mesh size of the honeycomb zeolite was 9-32. The uptake of Cs in mordenite reaches 83.97% at 20 min and 86.81% when equilibrium is reached at 1080 min in pure water medium and Cs uptake reaches 39.86% at 10 min and maximum 57.91% reaches at 15 hours after had been equilibrium is reached in seawater medium.
On the other hand, the uptake of Sr in zeolite type ‘A’ was found 98.08% within 10 min after getting contact and reaches maximum 99.18% at 1440 min in equilibrium. This indicates that the adsorption proceeds at fast within 20 min and slower rate when equilibrium reached in aqueous solution. In case of adsorption in seawater uptake of Sr in zeolite A was found to be 59.71% within 20 min and reaches maximum 69.01% at 180 min. This indicates that the adsorption for both the zeolites proceeds at fast within 10-20 min and slower rate when equilibrium is reached.
Mass uptake relationship
Batch adsorption experiments have been performed to clarify the selectivity of the exchange materials using ionic solution. The effect of solution volume to exchanger mass was investigated to determine the minimum weight of exchange material that would be able to provide a reasonable uptake percentage of Cs+ and Sr2 in seawater. A weighted amount of the exchange materials (32-48 mesh) 0.02 gm-0.666 gm were mixed with 10 ml ionic 10 ppm ionic solution of Cs and Sr mixed and shaken at room temperature 25°C for 24 h. Figure 2 shows the uptake percentage of Cs and Sr with respect to mass of the exchange material. The maximum removal percentage of Sr and Cs was found to be 92.16%, and 84.46% at V/m ratio=15 (ml/g) for mordenite and zeolite type ‘A’ respectively.
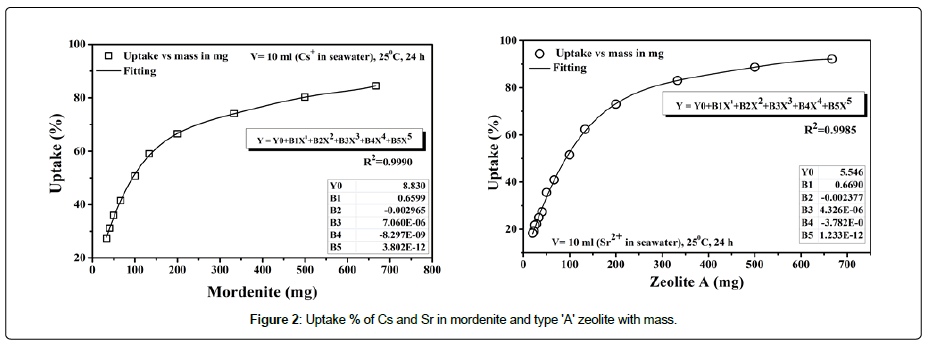
Figure 2: Uptake % of Cs and Sr in mordenite and type 'A' zeolite with mass.
Effect of temperature in adsorption
Temperature has a pronounced effect on the adsorption characteristics. An investigation of temperature on adsorption characteristics has been conducted for Cs+ and Sr2+ ions onto mordenite and type ‘A’ zeolite respectively from 25°C-70°C. From the Figure 3, it is shown that adsorption decreases with increasing temperature abruptly for Cs but in case of Sr, the nature of adsorption is little different with temperature which shows flat adsorption above 50°C. This can be explaining by exothermic spontaneity of adsorption process and by weakening of bonds between ions and active sites of the zeolite pores at high temperature.
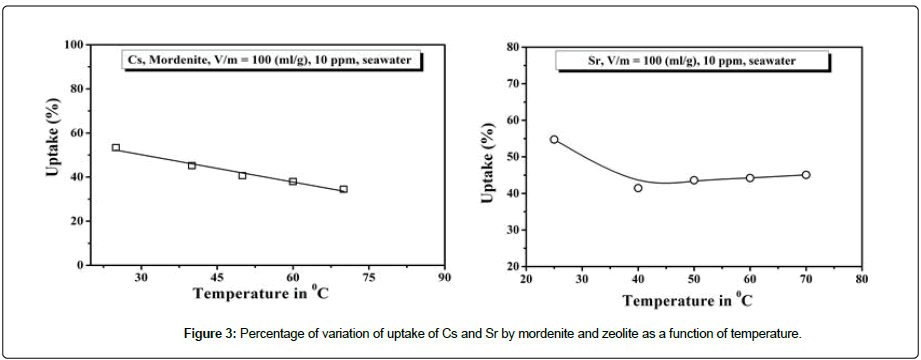
Figure 3: Percentage of variation of uptake of Cs and Sr by mordenite and zeolite as a function of temperature.
Effect of concentration
The adsorption isotherms were measured by gradually increasing the concentration of the sorbent ions at each equivalent concentration. The adsorption of the mordenite and zeolite type A have been conducted by increasing the concentration of Cs+ and Sr2+ respectively in the range of 1 mg/l-50 mg/l at a constant volume mass ratio adoption respectively have been studies with volume mass ratio of 300 ml/g. The Figure 4 shows that the percentage of adsorption decrease with increasing concentration of the solution increases and reaches nearly saturated which shows the logarithmic nature. This indicates that for a given mass of ion exchanging material the removal percentage is maximum, and no metal ions adsorbed further after the equilibrium of the concentration is reached.
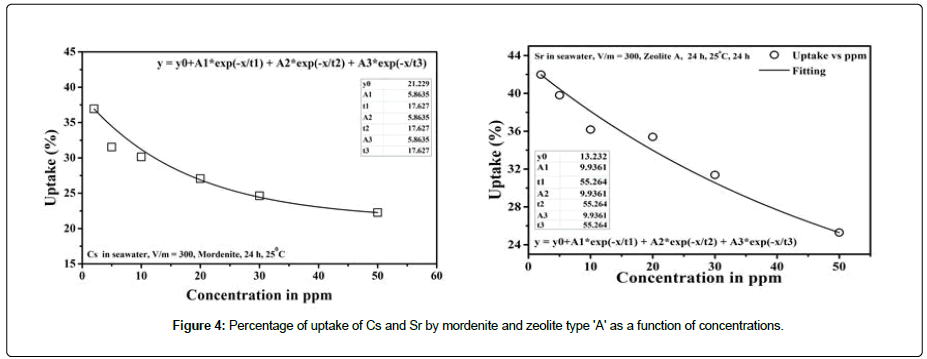
Figure 4: Percentage of uptake of Cs and Sr by mordenite and zeolite type 'A' as a function of concentrations.
Column studies
Batch experiment is often difficult to apply to column system because it sometimes unable to provide accurate data for scale up since flow in the column is not equilibrium. The sorption study of the zeolites is conducted with vertical column system which could provide the information on the efficient utilization for practical use. The experiment was carried out in a glass column and solution was passed in clock wise circulation. Two different flow rate 1 ml/min and 3 ml/ min respectively with initial concentration of 10 ppm for each ionic solution in seawater media was used. The adsorption characteristics of mordenite and zeolite type ‘A’ with time in the column for the Cs and Sr respectively are shown in Figure 5. The uptake of Cs in mordenite shows the increase with time and leads to be saturated about 133 hrs is reached which follows the polynomial nature. The percentage of adsorption reaches at 70.26% and 64.53% at 168 hrs in a flow rate of 1 ml/min and 2.82 ml/min respectively. On the other hand, in case Sr uptake in zeolite type ‘A’, shows the same nature of adsorption behavior with time follows the same nature of the fitting equation. The adsorption of Sr was found 52.69% and 71.16% at a flow rate of 1 ml/min and 3 ml/min respectively. It is seen that sorption rate is increased by flow rate indicating that mass dominating the overall system kinetics.
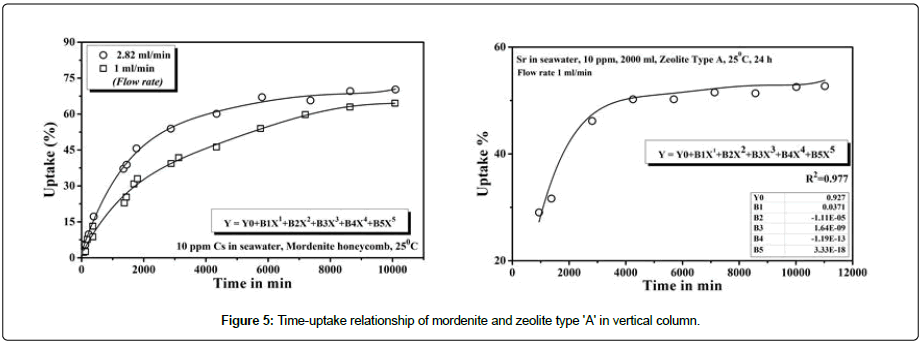
Figure 5: Time-uptake relationship of mordenite and zeolite type 'A' in vertical column.
Both the zeolite follows the polynomial nature of adsorption characteristics with time which can be represented by the equation below and parameters involved are given in Table 2
Table 2: Constants and correlation coefficient of polynomial equation in adsorption of Cs and Sr with time.
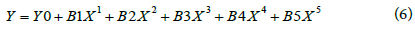
Solidifications
The development of stable solidification method of ion exchange material zeolite is an important task for the safety decontamination of 137Cs and 90Sr from liquid radioactive waste. By using the excellent immobilizing properties of zeolites such as gas trapping ability and self-sintering properties, the stable solidification was accomplished (Figures 6 and 7). By using the immobilization ability of zeolites, i.e., Cs and Sr trapping and self-sintering abilities, the discs of zeolite were sintered at higher temperatures up to 1200°C. The immobilization ratio of Cs for mordenite saturated with Cs+ ions was estimated to be less than 0.1% above 1200°C; the adsorbed Cs+ ions are completely volatilized but slightly melted above 1000°C. On the other hand, Sr2+ saturated was estimated to be 0.3% at temperature 1200°C is given in Table 3.
Table 3: EDS data of the mordenite and type ‘A’ zeolite after thermal treatment at different temperature.
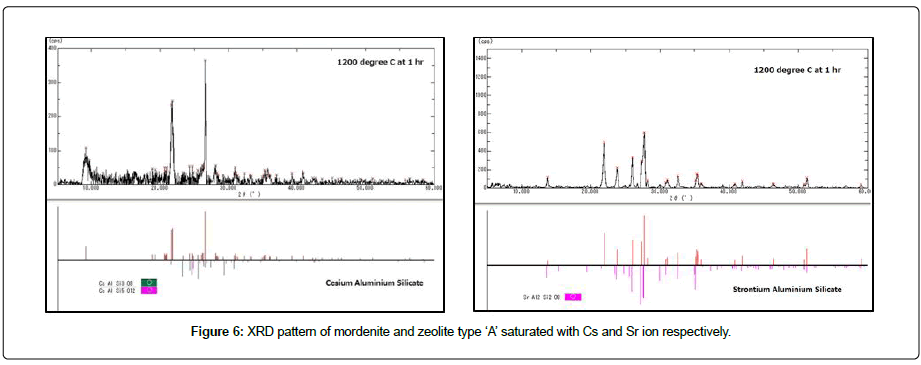
Figure 6: XRD pattern of mordenite and zeolite type ‘A’ saturated with Cs and Sr ion respectively.
Figure 7: SEM image of mordenite and type A zeolite at different temperature.
Conclusion
The adsorption characteristics of synthetic mordenite and type ‘A’ zeolite in the form of honeycomb have been studied as an inorganic ion exchange for the removal of Cs and Sr in presence of highly concentrated competing cations (Na) in seawater. The fibrous honeycomb zeolite was used to conduct vertical column system and hence the time dependence adsorption is established by mathematical equation. It is seen that the removal is strong and is a function of initial flow rate. Increase of removal efficiency with flow rate indicates that higher mass is more favorable for decontamination. Relatively, higher sorption capacity and column performance were obtained for Cs and Sr separation even in presence of high salt. This result shows that the mordenite and type ‘A’ zeolite in the form of honeycomb has an efficient nature in removing radionuclides i.e, of Cs and Sr from liquid waste water even at very high salt solution. The XRD pattern of the zeolite adsorptions of Cs on mordenite are recrystallized to CsAlSi3O6 and SrAl2Si2O8 at 1000°C and 1200°C respectively. These recrystallize phases are suitable hosts for the immobilization of Cs and Sr of the decontamination of radioactive water.
References
- International Atomic Energy Agency (1992) Handling and treatment of radioactive aqueous wastes, IAEA TECDOC 654, IAEA, Vienna.
- El-Kamash AM, Zaki AA, El-Geleel MA (2005) Modeling batch kinetic and thermodynamics of zinc and cadmium ions removal from waste solutions using synthetic zeolite A. J Hazard Mater 127: 211-220.
- Abd El-Rahman KM, El-Kamash AM, El-Sourougy MR, Abdel-Moniem NM (2006) Thermodynamic modeling for the removal of Cs+, Sr2+, Ca2+ and Mg2+ ions from aqueous waste solutions using zeolite A. J Radioanal Nucl Chem 268: 221-230.
- Barros MASD, Arroyo PA, Sousa-Aguiar EF, Tavares CRG (2004) Thermodynamics of the exchange processes between K+, Ca2+ and Cr3+ in Zeolite NaA. Adsorption 10: 227-235.
- Sinha PK, Lal KB, Panicker PK, Krishnasamy V (1996) A Comparative study on indigenously available synthetic zeolites for removal of strontium from solutions by ion-exchange. Radiochem Acta 73: 157-163.
- Dyer A, Abdel Gawad AS, Mikhail M, Enamy H, Afshang M (1991) The natural zeolite, laumontite, as a potential material for the treatment of aqueous nuclear wastes. J Radioanal Nucl Chem 154: 265-276.
- Ouki SK, Kavannagh M (1997) Performance of natural zeolites for the treatment of mixed metal-contaminated effluents. Waste Manage Res 15: 383-394.
- El-Kamash AM (2008) Evaluation of zeolite A for the sorptive removal of Cs+ and Sr2+ ions from aqueous solutions using batch and fixed bed column operations. J Hazard Mater 151: 432-445.
- Dyer A, Chimedtsogzol A, Campbell L, Williams C (2006) Uptake of caesium and strontium radioisotopes by natural zeolites from Mongolia. J Microporous and Mesoporous Materials 95: 172-175.
- Földesová M, LukáÄ P (1996) Leachability of cobalt and cesium from natural and chemically treated zeolites. J Radioanal Nucl Chem 214: 479-487.
- Dyer A, Las T, Zubair M (2000) The use of natural zeolites for radioactive waste treatment studies on leaching from zeolite/cement composites. J Radioanal Nucl Chem 243: 839-841.
- Osmanlioglu AE (2006) Management of spent sealed radioactive sources in Turkey. Health Phys 91: 258-262.
- El-Kamash AM, El-Naggar MR, El-Dessouky MI (2006) Immobilization of cesium and strontium radionuclides in zeolite-cement blends. J Hazard Materials 136: 310-316.
- Bagosia S, Csetenyia LJ (1999) Immobilization of caesium-loaded ion exchange resins in zeolite-cement blends. Cem Conc Res 29: 479-485
- Atun G, Bodur N (2002) Retention of Cs on zeolite, bentonite and their mixtures. J Radioanal Nucl Chem 253: 275-279.
- Christie T, Brathwaite B, Thompson B (2002) Mineral commodity report-23, Zeolite New Zealand Mining 31: 16-24.
- Abusafa A, Yücel H (2002) Removal of 137Cs from aqueous solutions using differ cationic forms of a natural zeolite: clinoptilolite. Separation Purificat Tech 28: 103-116.
- Jurado-Vargas M, Olguín MT, Erdóňez-Regil E, Miménez-Reyes M (1997) Ion exchange of radium and barium in zeolites. J Radioanal Nucl Chem 218: 153-156.
- Dyer A (1988) An Introduction to Zeolite molecular sieves, John Wiley and Sons, Bath Press Ltd., Avon, UK.
- Hamed MM, Attallah MF, Shehata FA (2012) Synthesis, Characterization and sorption behavior of some radionuclides on zirconium tungstate ion exchanger. Arab J Nucl Sci Appl 45: 37-50.
- Abd El-Rahman KM, El-Sourougy MR, Abdel-Momen NM, Ismail IM (2006) Modeling the sorption kinetics of cesium and strontium ions on zeolite A. J Nucl Radchem Sci 7: 21-27.
- Denton MS, Manos MJ, Kanatzidis MG (2009) Highly selective removal of cesium and strontium utilizing a new class of inorganic ion specific media, WM2009 Conference 1-5 March, Phoenix, AZ, USA.
- Mimura H, Kanno T (1985) Distribution and fixation of cesium and strontium in zeolite A and chabazite. J Nucl Sci Tech 22: 284-291.
- Mimura H, Kanno T (1982) Recrystallization of Cs and Sr form of synthetic mordenites and leachability of Cs and Sr from recrystallized products. J Atomic Engr Soci 24: 228-236.
- Mimura H, Kanno T (1981) Processing of radioactive waste solution with zeolites (II): Leachability of cesium and strontium from calcined zeolites, Science reports of the research institutes, Tohoku University. Ser. A. Physics, Chemistry and Metallurgy 30: 125-137.
- Tusa E, Harjula R, Lehto J (2003) Use of novel highly selective ion exchange media for minimizing the waste arising from different NPP and other liquids, WM'03 Conference, Tucson, AZ.
- Abdel Rahman RO, Ibrahium HA, Hung YT (2011) Liquid Radioactive Wastes Treatment: A Review. J Water 3: 551-565.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi