Short Communication, J Nucl Ene Sci Power Generat Technol Vol: 14 Issue: 1
Short Communication on Double Cross-Linked 3D Layered PBI Proton Exchange Membranes for Stable Fuel Cell Performance above 200℃
1College of Chemical Engineering, Zhejiang University of Technology, Hangzhou, China
2Institute of New Materials and Industrial Technologies, Wenzhou University, Wenzhou, China
*Corresponding Author: Lixin Xue,
College of Chemical Engineering, Zhejiang
University of Technology, Hangzhou, China
E-mail: zhangliang0721@163.com
Received date: 08 August, 2024, Manuscript No. JNPGT-24-144982;
Editor assigned date: 12 August, 2024, PreQC No. JNPGT-24-144982 (PQ);
Reviewed date: 26 August, 2024, QC No. JNPGT-24-144982;
Revised date: 02 September, 2024, Manuscript No. JNPGT-24-144982 (R);
Published date: 09 September, 2024, DOI: 10.4172/2325-9809.1000413.
Citation: Huang F and Xue L (2024) Short Communication on Double Cross-Linked 3D Layered PBI Proton Exchange Membranes for Stable Fuel Cell Performance above 200℃. J Nucl Ene Sci Power Generat Technol 13:5.
Abstract
Hydrogen fuel cells as an idea power source for new energy electric vehicles have been elevated to a national energy strategy level. The traditional hydrogen fuel cell technology route involves hydrogen, hydrogen refueling stations and fuel cells. However, issues such as the preparation of high-purity hydrogen, high-pressure storage,
transportation and the construction of refueling stations limit its widespread application and promotion.
Description
Hydrogen fuel cells as an idea power source for new energy electric vehicles have been elevated to a national energy strategy level. The traditional hydrogen fuel cell technology route involves hydrogen, hydrogen refueling stations and fuel cells. However, issues such as the preparation of high-purity hydrogen, high-pressure storage, transportation and the construction of refueling stations limit its widespread application and promotion. Methanol reforming hydrogen fuel cells use methanol as a liquid fuel which is convenient for storage and transportation allowing for “on-demand” hydrogen production. Methanol can be distributed and refueled using existing gas stations, making it safer and more reliable. If the reformer and proton exchange membrane fuel cell can operate at a temperature match (200℃-300℃), the high-temperature hydrogen-rich reformate generated from methanol can be used directly significantly simplifying the system and improving energy utilization efficiency. However, the currently used phosphoric acid-doped high-temperature proton exchange membranes are still limited to service temperatures of 120℃-200℃. At higher temperatures, issues such as phosphoric acid dehydration condensation and membrane mechanical creep can lead to decreased proton conductivity and cell performance. Therefore, overcoming this temperature limit and developing new high-temperature proton exchange membranes that can operate efficiently and stably above 200°C is key to achieving the field integration and direct use of methanol reforming systems and fuel cells.
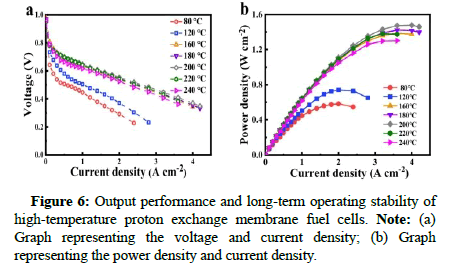
In this context, Fei Huang, professor from Zhejiang University of Technology, in collaboration with Lixin Xue, research scholar from Wenzhou University, has developed a gel-state phosphoric acid-doped polybenzimidazole (DC-PBI-G) high-temperature proton exchange membrane with a double cross-linked 3D layered structure using polyphosphoric acid sol-gel technology. This type of membrane material can effectively anchor and confine phosphoric acid molecules at high temperatures, successfully suppressing 96% of phosphoric acid dehydration condensation within the membrane, significantly improving mechanical creep resistance and providing excellent proton conductivity (0.348 S/cm) and stability [1]. Fuel cells based on DCPBI- G have demonstrated a peak single-cell power density of up to 1.20-1.48 W/cm² at 200℃-240℃, with a voltage degradation rate of only 0.27 mV/h over 250 hours of long-term operation, ultimately achieving efficient and stable utilization of high-temperature methanol reformate (Figures 1 and 2) [2].
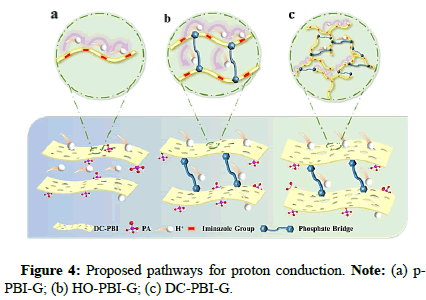
In this study, 3,3’-diaminobiphenyl (TAB), 2,5-dihydroxyterephthalic acid (DHTA) and Trimellitic Anhydride (TMA) were used as monomers to introduce HO-groups into the PBI network, forming cross-linked phosphoric acid bridges to in situ anchor phosphoric acid molecules and enhance proton conductivity. Simultaneously the addition of TMA branching monomers created a rigid branched network to improve the membrane’s creep resistance. Through the synergistic effect of this double cross-linked structure and film formation mechanism, a stable 3D layered Double cross-linked PAdoped gel-state PBI PEMs (DC-PBI-G) high-temperature proton exchange membrane was developed for high-temperature applications (Figures 3-5).
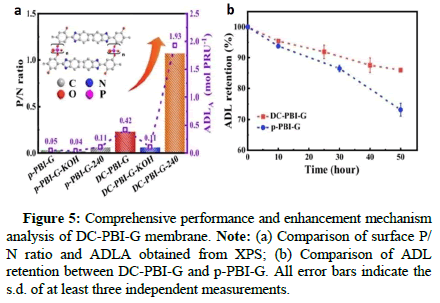
Figure 5: Comprehensive performance and enhancement mechanism analysis of DC-PBI-G membrane. Note: (a) Comparison of surface P/ N ratio and ADLA obtained from XPS; (b) Comparison of ADL retention between DC-PBI-G and p-PBI-G. All error bars indicate the s.d. of at least three independent measurements.
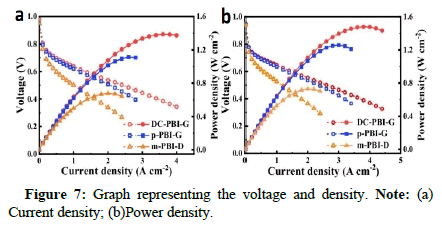
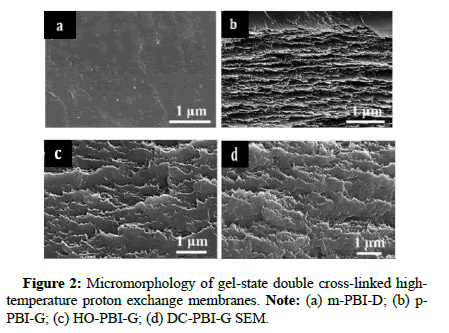
The authors conducted a comprehensive study on this membrane and various control groups obtained through different preparation methods, including gel-state HO-PBI-G with phosphoric acid bridges, non-cross-linked gel-state p-PBI-G and Dense-state M-PBI Membrane (m-PBI-D) prepared by traditional organic solvent casting. The study assessed structural, chemical and mechanical stability, phosphoric acid retention, proton conductivity and fuel cell performance.
The key to stable operation of the proton exchange membrane above 200℃ relies mainly on the DC-PBI-G membrane’s effective retention of phosphoric acid and significant improvement in creep resistance. The authors conducted 31P NMR and high-temperature acid retention tests on phosphoric acid-doped membranes. The results showed that at 240℃, DC-PBI-G successfully confined and anchored phosphoric acid molecules, effectively suppressing their dehydration condensation, with a retention rate as high as 96%. Additionally, compared to single-cross-linked and non-cross-linked membranes, the double-cross-linked DC-PBI-G has extra proton transport channels and a more continuous three-dimensional hydrogen bond network. The proton conductivity of DC-PBI-G reaches 0.348 S/cm at 220℃ and remains stable at 0.241 S/cm with a retention rate of 72% after 100 hours of testing at 240℃ (Figures 6-8).
Finally, the authors conducted comprehensive performance tests on high-temperature fuel cells based on the double-cross-linked DC-PBIG membrane. Under conditions of 200℃ and 240°C, the peak power densities reached 1480 mW/cm² and 1302 mW/cm², respectively (using hydrogen/oxygen, without backpressure and without additional humidification). Furthermore, the fuel cells based on this membrane also achieved an excellent peak power density of 636 mW/cm² under methanol reformate gas/oxygen conditions at 240℃ [3]. In hightemperature durability tests at 220℃, the voltage degradation rate of the DC-PBI-G membrane was significantly reduced compared to other membranes, with a degradation rate of only 0.27 mV/h after 250 hours of testing. The DC-PBI-G membrane also performed exceptionally well in low-temperature environments, with its fuel cell achieving a peak power density of 443 mW/cm² at 40℃ and maintaining a very low degradation rate of 8.97 μV/h. Therefore, the DC-PBI-G membrane demonstrates outstanding fuel cell performance and operational flexibility under both high and low-temperature conditions, marking a significant breakthrough in the development of hightemperature fuel cell technology [4].
Conclusion
In summary, the authors successfully developed a threedimensional layered DC-PBI-G proton exchange membrane by combining polyphosphoric acid sol-gel processing with a doublecross- linked network. This advancement significantly improves phosphoric acid stability, proton conductivity, creep resistance and fuel cell performance. This innovative research breaks through the conventional limitations of high-temperature proton exchange membrane fuel cells, providing new materials and methods for the development of high-performance proton membranes. It holds promise for addressing industrial development bottlenecks related to hydrogen production, storage, transportation and refueling and has scientific significance and application value for achieving national “dual carbon” strategic goals.
Funding
This research was funded by the National Natural Science Foundation of China (No. NSFC-22209147) and the strategic research and consulting project of the Chinese Academy of Engineering (No. 2022-DZ-08).
References
- Sannigrahi P, Ingall E (2005) Polyphosphates as a source of enhanced P fluxes in marine sediments overlain by anoxic waters: Evidence from 31P NMR. Geochem Trans 6:1-8.
- Aihara Y, Sonai A, Hattori M, Hayamizu K (2006) Ion conduction mechanisms and thermal properties of hydrated and anhydrous phosphoric acids studied with 1H, 2H and 31P NMR. J Phys Chem B110(49):24999-5006.
- Chen X, Qian G, Molleo MA, Benicewicz BC, Ploehn HJ (2015) High temperature creep behavior of phosphoric acid‐polybenzimidazole gel membranes. J Polym Sci 53(21):1527-1538.
- Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, et al. (1995) A smooth particle mesh Ewald method. J Chem Phys 103(19):8577-8593.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 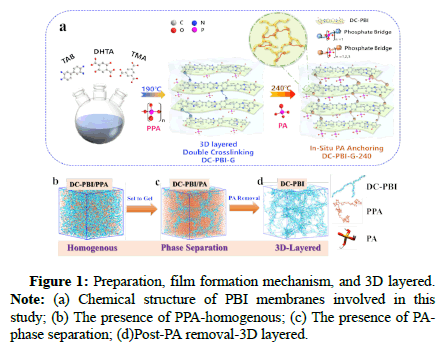
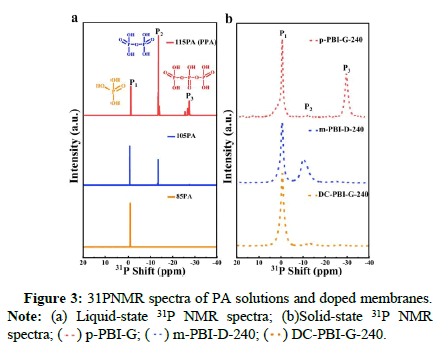
 ) p-PBI-G; (
) p-PBI-G; ( ) m-PBI-D-240; (
) m-PBI-D-240; ( ) DC-PBI-G-240.
) DC-PBI-G-240.