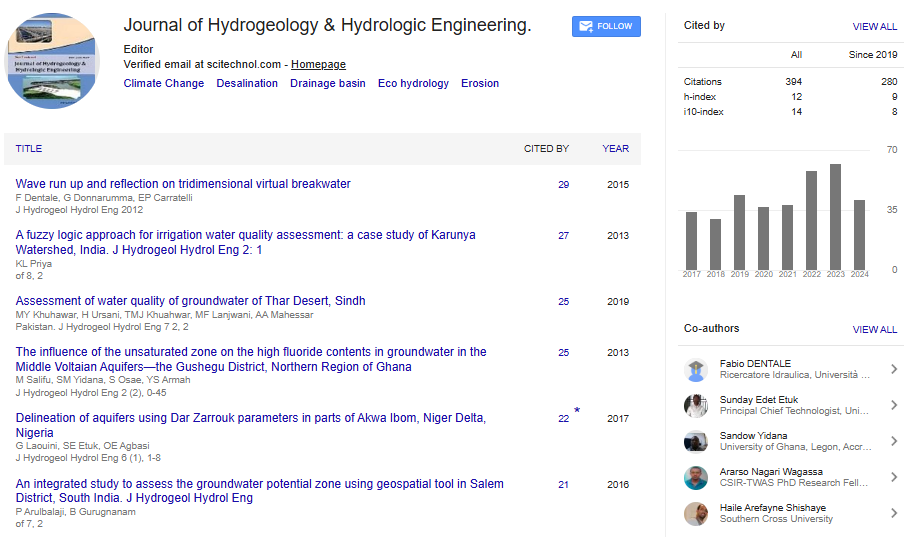
Perspective, J Hydrogeol Hydrol Eng Vol: 12 Issue: 4
Stable Isotopes and Radioactive Isotopes in Hydrology
Laura Garcia*
1Department of Geological Sciences, University of Alabama, Tuscaloosa, United State of America
*Corresponding Author: Laura Garcia,
Department of Geological Sciences,
University of Alabama, Tuscaloosa, United State of America
E-mail: garcial57@gmail.com
Received date: 17 July, 2023, Manuscript No. JHHE-23-114464;
Editor assigned date: 19 July, 2023, PreQC No. JHHE-23-114464 (PQ);
Reviewed date: 03 August, 2023, QC No. JHHE-23-114464;
Revised date: 11 August, 2023, Manuscript No. JHHE-23-114464 (R);
Published date: 21 August, 2023, DOI: 10.4172/2325-9647.1000276
Citation: Garcia L (2023) Stable Isotopes and Radioactive Isotopes in Hydrology. J Hydrogeol Hydrol Eng 12:4.
Description
Hydrology, the study of water and its movement in the Earth's atmosphere and on its surface, plays a essential role in understanding various natural processes, from weather patterns to ecosystem dynamics. Stable isotopes and radioactive isotopes are invaluable tools in hydrology, offering unique insights into the origin, movement, and age of water in different environments. This article explores the significance of stable and radioactive isotopes in hydrology and their applications in unraveling the mysteries of Earth's water cycle.
Stable isotopes are non-radioactive isotopes of an element that have the same number of protons but a different number of neutrons in their nuclei. Hydrologists often use stable isotopes of Hydrogen (H), Oxygen (O), and Carbon (C) to investigate various aspects of the hydrological cycle.
Water molecules consist of two hydrogen atoms and one Oxygen Atom (H2O). The ratio of heavy hydrogen isotope deuterium (2H) to regular hydrogen (1H) and the ratio of the heavy oxygen isotope Oxygen-18 (18O) to the more common Oxygen-16 (16O) vary in water molecules depending on their source and environmental conditions. These variations provide valuable information about the source, movement, and temperature of water.
By analyzing the isotopic composition of precipitation, hydrologists can trace the sources of water in a particular region. For example, the isotopic signature of rainwater in a region can reveal whether it originated from local evaporation, remote ocean sources, or even continental ice caps. This information is essential for managing water resources in areas with limited water availability.
Stable isotopes are also used to study groundwater flow. By comparing the isotopic composition of groundwater samples taken at different depths or locations, hydrologists can infer the direction of groundwater flow and identify potential sources of contamination.
Carbon isotopes, particularly Carbon-13 (13C) and Carbon-14 (14C), can provide insights into the age and origin of dissolved organic matter in aquatic systems. Radiocarbon dating, which relies on the radioactive decay of carbon-14, is used to determine the age of ancient water sources and the rate of groundwater recharge. This information is essential for managing groundwater resources and assessing the vulnerability of aquifers to contamination.
While stable isotopes offer valuable information about the composition and movement of water, radioactive isotopes provide insights into the dynamics of water flow and transport processes.
Tritium, a radioactive isotope of hydrogen, is produced in the atmosphere through nuclear reactions. It can be used as a tracer to study the movement of water in the subsurface. By measuring tritium concentrations in groundwater samples, hydrologists can determine the age of groundwater and assess the rates of groundwater recharge and discharge. Tritium has been particularly useful in identifying the impact of human activities on groundwater systems.
Radon is a naturally occurring radioactive gas that is released from the Earth's layer and dissolves in groundwater. Monitoring radon concentrations in groundwater can help identify the sources of groundwater contamination and assess the potential risks to human health.
Iodine-129 is a long-lived radioactive isotope that can be used to trace the movement of water in deep geological formations, such as those associated with nuclear waste repositories. Its relatively long half-life makes it a valuable tracer for studying groundwater flow over extended periods.
Stable and radioactive isotopes have revolutionized the field of hydrology by providing powerful tools for studying water sources, movement, and age. These isotopes are essential for managing water resources, protecting groundwater quality, and understanding the impacts of climate change on hydrological processes.
In the future, advances in analytical techniques, such as mass spectrometry, will continue to enhance our ability to measure isotopic ratios with high precision. This will enable hydrologists to answer increasingly complex questions about water cycle dynamics, including the effects of climate change, land use, and pollution on water resources.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi