Research Article, J Clin Nutr Metab Vol: 3 Issue: 2
The Effect of Flaxseed Powder and Oil on Reduction of Hyperglycemia and Associated Parameters in StreptozotocinInduced Diabetic Rats
Suha Hashim Abduljawad*
Department of Nutrition and Food Science, Faculty of Family Science, Taibah University, Al-Madinah Al-Munawarah, Kingdom of Saudi Arabia
*Corresponding Author : Suha Hashim Abduljawad
Department of Nutrition and Food Science, Faculty of Family Science, Taibah University, Al-Madinah Al-Munawarah, Kingdom of Saudi Arabia
E-mail: suha_hashem@yahoo.com
Received: February 08, 2019 Accepted: February 15, 2019 Published: February 22, 2019
Citation: Abduljawad SH (2019) The Effect of Flaxseed Powder and Oil on Reduction of Hyperglycemia and Associated Parameters in Streptozotocin-Induced Diabetic Rats. J Clin Nutr Metab 3:1. doi: 10.4172/JCNM.1000125
Abstract
The aim of this research was to compare the 10% flaxseed powder and 4% flaxseed oil effects on reduction of hyperglycemia and associated parameters streptozotocin (STZ) induced diabetic rats. Diabetes mellitus was induced in Sprague-Dawley rats using streptozotocin. The rats were divided into six groups consisting of 5 rats in each group. C, control; CD, diabetes control; CFP, normal rats feed on diet contain 10% flaxseed powder; CFO, normal rats feed on diet contains 4% flaxseed oil; DFP, diabetic rats feed on diet contain 10% flaxseed powder; DFO, diabetic rats fed on a diet containing 4% flaxseed oil. The highest significant increase (p<0.01) of Total glutathione levels and Superoxide dismutase and Catalase activity were seen in group CFP when compared to CD group. Supplementation with flaxseed powder in the diet was more effective than flaxseed oil in the reduction of blood glucose level of diabetic rats. DFP group showed the lowest significant (P<0.05) decreasing in total cholesterol, Triglycerides and low-density lipoprotein, and the highest significant (P<0.05) increasing in high-density lipoprotein as compared to diabetic CD group. Conclusion: Diabetic rats in a group which were treated with 10% flaxseed powder were able to partly recover from STZ-induced diabetes within eight weeks when compared to DC group.
Keywords: Flaxseed powder, Flaxseed oil, Hyperglycemia, Antioxidants, Lipid profile
Introduction
Diabetes mellitus is a metabolic disorder which affects millions of people worldwide. It is characterized by hyperglycemia that results either from an absolute or relative insulin deficiency. It is associated with long-term complications [1]. Recent studies have reported that patients with diabetes have a high susceptibility for numerous liver diseases, including non-alcoholic fatty liver disease, abnormal liver enzymes, acute liver failure, cirrhosis, as well as hepatocellular carcinoma [2], cardiovascular, nephropathic and neuropathic diseases [3]. Diabetics who suffer from metabolic disorders like hypertension and hyperlipidemia are easily exposed to cardio-cerebrovascular diseases, for example, coronary heart disease which is a risk factor that often leads to death [4]. Therefore, it is critical to establish strategies to prevent and control the epidemic trend of diabetes mellitus and its complications [5].
The treatment of diabetes mellitus depends enormously on dietary measures, which may include the use of traditional plant therapies. Linum usitatissimum or flaxseed or linseed belongs to family Linaceae. Flaxseeds contain flax oil which contains unsaturated fatty acids such as oleic acid (12–30%), linoleic acid (8–29%) as well as linolenic acid (35–67%) [4]. These particular fatty acids have been found to have significant anti-inflammatory [6], and antiulcer properties [7]. Flaxseed and its oil are among the top bestselling herbal dietary supplements [8]. It also contains plant lignan secoisolariciresinol diglycoside (SDG). The SDG from L. usitatissimum known as antioxidant [9], antihyperlipidemic, antihypercholesterolemic, antihyperinsulenaemic and antihyperlipidaemic [10]. SDG and omega-3 fatty acids are well known for improving hyperlipidemia. Generally, flaxseeds are known to minimize the severity of diabetes by making blood glucose levels stable [11].
In addition to the glucoregulatory benefits of flaxseeds, studies have proved a cardioprotective role through improved arterial compliance as well as anticoagulant properties [12,13].
Many studies have shown flaxseed’s beneficial effects. Therefore, the protective effects of flaxseeds and flaxseeds oil on the complications of diabetes is worth studying. The present study was carried out to assess both the complications induced by diabetes and the protective effects of flaxseeds supplements on wistar rats.
Materials and Methods
Chemicals
Streptozotocin (STZ) was purchased from Sigma-Aldrich, Milwaukee, USA. All the other chemicals used in this research were of analytical grade.
Plant material
Flaxseeds with a moisture content of 6.2 wt% were purchased from the local co., Menoufia, Egypt. They were cleaned in a careful manner by hand to remove the foreign materials such as other seeds, stones, and small stalks. The cleaned flaxseeds were dried for twelve hours at 105°C in an oven, and then crushed into powder in a grinder (B-400, Buchi Labortechnik AG, Switzerland) with a size range of 0.45–1.2 mm. The powder was kept in a vacuum dryer until approaching time of use.
Crude extracted flaxseeds oil was freshly purchased from Awara Co., Tanta, Egypt. The oil was stored in dark glass bottles at a temperature ranging from 15°C and 20°C. The flaxseeds oil contains 30%-40% fixed oil which includes linoleic, oleic, palmitic and stearic acids. It also contains mucilage (6%), protein (25%), the cyanogenic glycoside linamarin, bitter, minerals and amino acids. It also contains vitamins A, B, D and E [14].
Animals, diets and experimental protocol
Thirty male albino rats (Sprague-Dawley strain) weighing 140 ± 10 g and aged between 8-10 weeks old were accessed from The Holding Company for Biological Products and Vaccines (VACSERA, KSA, Riyadh). The rats were kept in wire-bottomed cages in a room under a standard condition of illumination with a 12 – hours light-dark cycle at 25 ± 1°C. The rats were provided with tap water and a balanced diet. The experiments were approved by the state authorities and it also followed the Egyptian rules on animal protection.
The standard diet according to Reeves et al.[15] consisted of casein (12%), corn oil (10%), cellulose (5%), salt mixture (4%), vitamin mixture (1%) [16], methionine (0.3%), choline chloride (0.2%) and cornstarch (up to 100%). For the flaxseed powder diet, the standard diet was mixed with 10% w/w flaxseed powder. As for the flaxseed oil diet, the standard diet was mixed with 4% w/w flaxseed oil. The feeds were prepared weekly, they were packed in individual plastic sealed bags in quantities sufficient for a day's feed and stored at-20°C.
After the adaptation period, the rats were divided into six groups of five rats each randomly. Out of the six groups, three were non-diabetic, while three were diabetic. The non-diabetic rats' groups were as follows: group C "negative control rats which were fed a standard diet"; group CFP "control rats, which were fed a 10% w/w flaxseed powder diet"; group CFO "control rats feed on 4% w/w flaxseed oil diet". The diabetic groups were termed as follows: group DC "diabetic rats (positive control), which were fed a standard diet"; group DFP "diabetic rats, which were fed a 10% w/w flaxseed powder diet"; group DFO "diabetic rats, which were fed a 4% w/w flaxseed oil diet". The diets and fresh water were provided as they liked. Daily feed consumptions were recorded throughout the experimental period (8 weeks), body weight and feed efficiency ratio (FER) was recorded according to the methods of Chapman et al. [16].
The diabetic rats were developed by multiple low-dose STZ injections based on previous reports with some modifications. Rats were weighed and ear-notched. STZ was dissolved in cold 0.01 M citrate buffer, pH 4.5, which was always freshly prepared for immediate use. STZ injections were administered intraperitoneally (i.p.), and the doses were determined according to the body weights of the animals. Fifteen rats were administered CsA (20 mg/kg/day, s.c.) daily for 10 days prior to the STZ treatment and simultaneously received multiple low doses of streptozotocin (MLDSTZ) (20, 40 and 60 mg/kg/day, i.p.) for 5 consecutive days (5 rats/group. Non-fasting blood samples were collected twice per week by tail bleeding into heparinized tubes. The glucose concentrations in the plasma samples were obtained using the enzymatic colorimetric method. The rats were defined as diabetics when their non-fasting blood glucose level (BGL) reached more than 200 mg/dl in two consecutive readings [17,18].
Assessment of antioxidant activity
After 8 weeks, the rats were deprived of food for 14 hours and then scarified under ether anesthesia. The abdomen was opened and blood was collected from the abdominal aorta. Blood samples were centrifuged at 3000 rpm for 7 min and the clear serum was stored at −20°C until analysis. Liver, heart, and kidney were removed and blotted. Heart, liver, and kidney were exposed and perfused with cold phosphate buffer saline of pH 7.4. Blood free tissues were homogenized and subjected to following estimations. Total glutathione (GSH) was evaluated by the method of Sedlak and Lindsay [19]. Superoxide dismutase (SOD) was measured by using the method of Ellmann (1958) Catalase (CAT) activity was evaluated using the method of Claiborne [20].
Determination of serum blood glucose level
Serum glucose level was measured according to the method of Young [21] by using glucose kits (Spinreact, Spain).
Estimations of Lipid profile
Serum Total Cholesterol (TC), Triglycerides (TG) and High-Density Lipoprotein cholesterol (HDL-C) were measured by using reagents and kits available (Merck KGaA, Darmstadt, Germany). The amounts of Low-Density Lipoprotein cholesterol (LDL-C) were calculated with the help of the equation of Friedwald [22]:
Statistical analysis
All the data are presented as mean ± SD and analyzed by One-way ANOVA followed by Dunnett test for the possible significance identification between the numerous groups. p<0.05 was considered statistically significant. Statistical analysis was done using the Statistical Package for the Social Sciences (SPSS for WINDOWS, version 16.0; SPSS Inc, Chicago).
Results
The effect of flaxseed powder and flaxseed oil on body weight gain (BWG), food intake (FI) and feed efficiency ratio (FER) in normally fed rats on a diet containing 10% flaxseed powder as well as on diabetic rats fed on diet with 4% flaxseed oil are presented in Table 1. The mean values of BWG (g), FI (g/day) and FER for experimental group that were fed on basal diet only (C) were 4.10 ± 0.21, 18.75 ± 0.28 and 0.219 ± 0.007, respectively, while BWG (g), FI(g/day) and FER of diabetic group (CD) were 3.75 ± 0.35, 18.35 ± 0.40 and 0.204 ± 0.009, respectively. Results revealed that diabetic rats showed a significant (p<0.05) reduction in BWG (g) (3.75 ± 0.35) comparing to the control group (C) (4.10 ± 0.21). Moreover, Data showed that BWG (g) were decreased significantly (P<0.05) in DFP and DFO groups (3.00 ± 0.28 and 3.80 ± 0.20, respectively) comparing to C groups.
| Treatments(€) Parameter(→) | BWG (g) |
FI (g/day) |
FER |
|---|---|---|---|
| C | 4.10 ± 0.21a | 18.75 ± 0.28a | 0.219 ± 0.007a |
| DC | 3.75 ± 0.35b | 18.35 ± 0.40a | 0.204 ± 0.009a |
| CFP | 4.15 ± 0.25a | 18.70 ± 0.33a | 0.222 ± 0.012a |
| CFO | 4.05 ± 0.23a | 18.57 ± 0.37a | 0.218 ± 0.015a |
| DFP | 3.00 ± 0.28b | 18.60 ± 0.45a | 0.215 ± 0.020a |
| DFO | 3.80 ± 0.20b | 18.42 ± 0.52a | 0.206 ± 0.028a |
| Values expressed as mean ± SD. Values at the same column with different letters are significant at P<0.05 by Dunnet’s multiple comparison test C: control, CD: diabetes control, CFP: 10% flaxseed powder, CFO: 4% flaxseed oil, DFP: diabetes+10% flaxseed powder, DFO: diabetes+4%flaxseed oil |
|||
Table 1: Effect of flaxseed powder and flaxseed oil on mean values of body weight gain (BWG), food intake (FI) and feed efficiency ratio (FER) in male diabetic rats.
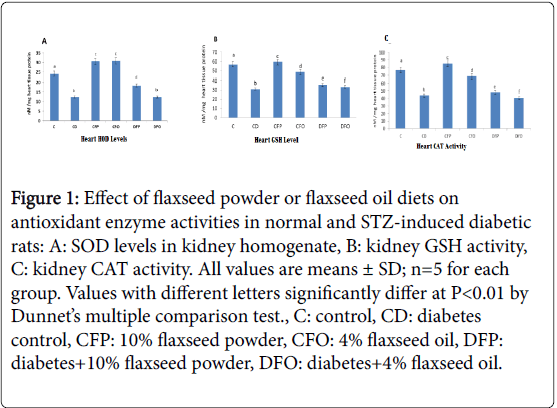
Antioxidant enzyme activities (CAT and SOD) and GSH levels in the heart homogenate of control and tested groups emerge in Figure 1(A-C). A Significant (p<0.01) decrease in GSH levels and SOD, CAT activity (nM/mg heart tissue protein) was seen in diabetic control (CD) (30.80 ± 0.74, 12.45 ± 0.85 and 43.83 ± 0.21, respectively) in comparison to normal control (C) (56.82 ± 0.37, 24.33 ± 0.46 and 76.67 ± 0.38, respectively). On the other hand, feeding diabetic rats on a diet containing 10% flaxseed powder (DFP) showed Significant (p<0.01) increase in GSH levels and SOD, CAT activity (nM/mg heart tissue protein) (35.59 ± 0.43, 18.35 ± 1.12 and 48.09 ± 050, respectively) in comparison to diabetic control (CD) (30.80 ± 0.74, 12.45 ± 085 and 43.83 ± 0.21, respectively).
Figure 1: Effect of flaxseed powder or flaxseed oil diets on antioxidant enzyme activities in normal and STZ-induced diabetic rats: A: SOD levels in kidney homogenate, B: kidney GSH activity, C: kidney CAT activity. All values are means ± SD; n=5 for each group. Values with different letters significantly differ at P< 0.01 by Dunnet’s multiple comparison test., C: control, CD: diabetes control, CFP: 10% flaxseed powder, CFO: 4% flaxseed oil, DFP: diabetes+10% flaxseed powder, DFO: diabetes+4% flaxseed oil.
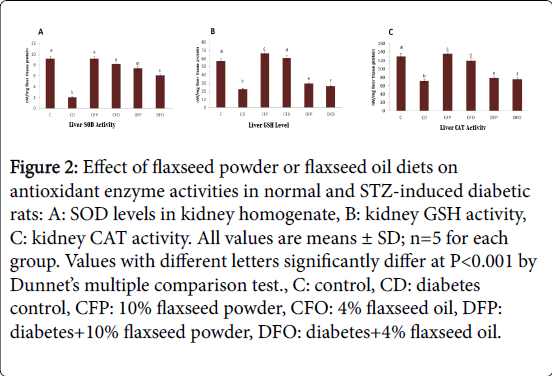
Figure 2(A-C) clarifies the enzyme activities (CAT and SOD) and GSH levels in the liver homogenate of control and tested groups. The mean value of GSH levels and, SOD and CAT activity (nM/mg liver tissue protein) for the experimental group (C) that fed on basal diet only were 57.23 ± 0.55, 8.19 ± 0.16 and 129.61 ± 0.62, respectively, while GSH levels and, SOD and CAT activity for the diabetic rat (CD) were 22.52 ± 0.31, 2.08 ± 0.18 and 71.30 ± 0.62, respectively. Results revealed that rats with CD group have a significant increase (p<0.01) in GSH levels and, SOD and CAT activity when compared with the C group. In the same figure, data demonstrated that feeding diabetic rat on diet containing 10% flaxseed powder (DFP) or 4% flaxseed oil showed Significantly (p<0.01) increase in GSH levels and, SOD and CAT activity (29.60 ± 0.59, 7.38 ± 0.54 and 78.48 ± 0.42, or 26.48 ± 0.56, 6.08 ± 0.73 and 75.44 ± 0.15, respectively) in comparison to diabetic control (CD).On the other hand, the highest significant increase (p<0.01) of GSH levels and, CAT activity of tested groups were seen in the CFP when compared to C group.
Figure 2: Effect of flaxseed powder or flaxseed oil diets on antioxidant enzyme activities in normal and STZ-induced diabetic rats: A: SOD levels in kidney homogenate, B: kidney GSH activity, C: kidney CAT activity. All values are means ± SD; n=5 for each group. Values with different letters significantly differ at P< 0.001 by Dunnet’s multiple comparison test., C: control, CD: diabetes control, CFP: 10% flaxseed powder, CFO: 4% flaxseed oil, DFP: diabetes+10% flaxseed powder, DFO: diabetes+4% flaxseed oil.
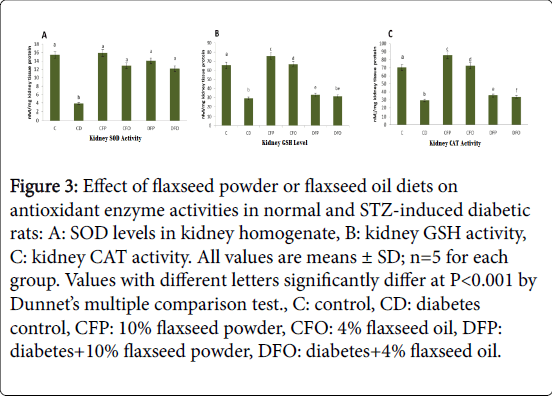
Figure 3(A-C) accentuates the activities of various antioxidant enzymes in kidney homogenate of rats in different groups. Diabetic rats (CD) showed significant (P<0.01) reduction in GSH levels and SOD and CAT activity (nM/mg kidney tissue protein) (28.97 ± 0.39, 3.88 ± 0.58 and 29.40 ± 0.33, respectively) as compared to control (C) (65.54 ± 0.27, 15.44 ± 1.21and 70.62 ± 0.37, respectively). in the same figure, a significant (P<0.01) restoration of the SOD and CAT activity were seen in both diabetic rats that were receiving 10% flaxseed powder (DFP) (14.05 ± 1.94 and 35.50 ± 0.33 nM/mg kidney tissue protein) and 4% flaxseed oil (DFO) (12.19 ± 0.15 and 33.76 ± 0.17 nM/mg kidney tissue protein) as compared to diabetic group (CD). Flaxseed powder diet (CFP) show the highest significant (P<0.01) elevation in renal GSH levels and CAT activity (75.38 ± 0.12 and 85.69 ± 0.50 nM/mg kidney tissue protein) as compared to control rats (C).
Figure 3: Effect of flaxseed powder or flaxseed oil diets on antioxidant enzyme activities in normal and STZ-induced diabetic rats: A: SOD levels in kidney homogenate, B: kidney GSH activity, C: kidney CAT activity. All values are means ± SD; n=5 for each group. Values with different letters significantly differ at P< 0.001 by Dunnet’s multiple comparison test., C: control, CD: diabetes control, CFP: 10% flaxseed powder, CFO: 4% flaxseed oil, DFP: diabetes+10% flaxseed powder, DFO: diabetes+4% flaxseed oil.
Results of blood glucose levels (mg/dl) are presented in Table 2. Diabetic group (CD) showed significant (P<0.05) increase in blood glucose level (295.42 ± 14.10) compared to healthy rats (86.28 ± 5.96). Results obtained from this table stated that a significant decreasing (P<0.05) in the mean values of blood glucose level (mg/dl) in DFP group (233.37 ± 12.95) compared with the diabetic group (CD). Generally, these results demonstrated that supplementation with flaxseed powder in the diet was more effective than flaxseed oil in the reduction of blood glucose level of diabetic rats.
| Treatments (€) Parameter (→) | Blood glucose (mg/dl) |
|---|---|
| C | 86.28 ± 5.96c |
| DC | 295.42 ± 14.10a |
| CFP | 84.77 ± 3.50c |
| CFO | 85.80 ± 7.21c |
| DFP | 233.37 ± 12.95b |
| DFO | 274.70 ± 15.86a |
| Values expressed as mean ± SD. Values at the same column with different letters are significant at P<0.05 C: control, CD: diabetes control, CFP: 10% flaxseed powder, CFO: 4% flaxseed oil, DFP: diabetes+10% flaxseed powder, DFO: diabetes+4%flaxseed oil |
|
Table 2: Effect of flaxseed powder and flaxseed oil on blood glucose level (mg/dl) in male diabetic rats.
The effect of flaxseed powder and flaxseed oil on total Cholesterol (TC), Triglycerides (TG), high-density lipoprotein cholesterol (HDLC) and low-density lipoprotein cholesterol (LDL-C) in normal fed rats on a diet containing 4% and 10% flaxseed powder as well as in diabetic rats fed on a diet with 4% and 10% flaxseed oil are presented in Table 3. Data showed that mean values of TC, TG, HDL-C and LDL-C (mg/dl) for the experimental group that was fed on basal diet only (C) were 79.1 ± 3.6, 100.2 ± 4.2, 23.0 ± 2.0 and 36.1 ± 2.8, respectively, while TC, TG, HDL-C and LDL-C (mg/dl) of the diabetic group (CD) were 98.8 ± 4.7, 164.5 ± 8.3, 19.4 ± 2.0and 46.5 ± 4.2, respectively. Results revealed that diabetic rats showed a significant (p<0.05) increase in TC, TG, and LDL-C when compared to the control group (C). On the other hand, mean values of HDL-C were significantly (P<0.05) decreased in the diabetic group (CD) (19.4 ± 2.0 mg/dl) as compared to C (23.0 ± 2.0 mg/dl). The dietary supplementation of 10% flaxseed powder in group CFP showed that TC, TG, and LDL-c levels were the lowest significant (p<0.05) reduction among all treated groups compared with control group (C), also these treatments led to The highest significant (p<0.05) increase in HDL-c among all treated groups compared with control group (C).
| Parameters (→) Treatments (€) |
Cholesterol (mg/dl) |
Triglycerides (mg/dl) |
HDL (mg/dl) |
LDL (mg/dl) |
|---|---|---|---|---|
| C | 79.1 ± 3.6c | 100.2 ± 4.2c | 23.0 ± 2.0c | 36.1 ± 2.8c |
| DC | 98.8 ± 4.7b | 164.5 ± 8.3b | 19.4 ± 2.0b | 46.5 ± 4.2d |
| CFP | 72.3 ± 3.5a | 90.5 ± 3.8a | 25.2 ± 1.9a | 29.0 ± 2.6a |
| CFO | 75.5 ± 3.9c | 99.1 ± 4.4c | 23.3 ± 2.1c | 32.4 ± 2.8b |
| DFP | 82.5 ± 4.0c | 132.0 ± 7.2d | 22.8 ± 1.7d | 33.3 ± 3.7b |
| DFO | 92.2 ± 4.8d | 157.0 ± 8.6b | 19.7 ± 1.9b | 41.1 ± 4.3c |
| Values expressed as mean ± SD. Values at the same column with different letters are significant at P<0.05 C: control, CD: diabetes control, CFP: 10% flaxseed powder, CFO: 4% flaxseed oil, DFP: diabetes+10% flaxseed powder, DFO: diabetes+4%flaxseed oil. |
||||
Table 3: Effect of flaxseed powder and flaxseed oil on lipid profile in male diabetic rats.
On the other hand, the diabetic group that fed on a diet containing 10% flaxseed powder as treatment (DFP) showed significant (P<0.05) decrease in TC, TG, and LDL-C, and significant (P<0.05) increase in HDL-C as compared to CD group. Also, results obtained from these tables stated that the diabetic group which was fed on a diet containing 4% flaxseed oil (DFO) as treatment showed significant (P<0.05) decrease in TC and LDL-C, as compared to diabetic CD group.
Discussion
Natural herbal dietary supplements that are capable of modifying diabetes and hyperlipidemia become more popular and appealing to patients [23]. These herbal supplements include; Linum usitatissimum L.-the richest known source of α-linolenic acid (ALA, omega-3 fatty acid)-lignans, and dietary fiber [24], which could potentially lead to effects in the prevention of some chronic diseases such as; mitigating the effects of diabetes, cardiovascular diseases, renal pathologies, obesity, colon and rectum cancer, reducing serum cholesterol level, and promoting the intestinal evacuation [25].
This current study was performed to compare the flaxseed powder and oil effects on reduction of hyperglycemia and associated parameters in streptozotocin (STZ) induced diabetic rats. It is known that STZ produces hyperglycemia in rats. In this study, the cytotoxic action of STZ in diabetic rats (DC) produces significant (P<0.05) decrease of BWG, when compared to the C group. Moreover, both administration of DFP and DFO significantly (P<0.05) decrease BWG. Swanston-Fiatt et al. [26] explained that; induction of diabetic by STZ is associated with characteristic weight loss due to muscle wasting and catabolism of tissue proteins.
One of the principal causes of diabetes-induced injury is the formation of lipid peroxides by free radical derivatives [27]. The body has an effective defense mechanism to prevent and to neutralize the free radical-induced damage. This is proficient by a set of endogenous antioxidant enzymes such as SOD and CAT. These enzymes constitute a mutually supportive team of defense against ROS [28]. In diabetes, the balance between ROS production and these antioxidant defenses may be lost, resulting in oxidative stress and tissue inflammation. The reduced activities of SOD and CAT point out the tissues damage in the diabetic rats. A significant increase (P<0.01) in SOD and CAT activities in heart, liver, and kidney homogenate tissues were observed in DFP group as compared to the DC group. This indicates the antioxidant activity of the flaxseed Powder.
Regarding non-enzymic antioxidants, GSH is a critical determinant of tissues susceptibility to oxidative damage and the depletion of GSH has been shown to be associated with diabetic status [29]. The present study revealed a significant increase (P<0.01) in GSH level in heart, liver, and kidney homogenate tissues were observed in DFP group as compared to the DC group, the increase in GSH level may be due to the GSH synthesis or GSH regeneration, while these results disagree with Hussein et al. [30] who indicates that Flaxseed oil as a source of omega-3 fatty acids (mainly α-linolenic acid) enhanced omega-3 fatty acids to compete with omega-6 family for incorporation into cell membrane resulting in a reduction of arachidonic acid (omega-6) liberation from the cell membrane [31]. The reduction of arachidonic acid leads to a reduction of reactive oxygen species (ROS) and increasing antioxidant enzymes, while Pilar et al. [31] indicated that both flaxseed oil and flaxseed powder can positively affect enzymatic antioxidant defenses both in healthy and diabetic groups. Moreover, Han et al. [32] suggested that fiber flaxseeds would be valuable candidates as functional products and dietary supplements production owing to the higher bioactive values as well as flaxseeds oil.
In the present study, a positive correlation between hyperglycemia and hyperlipidemia was obtained. In diabetic rats (CD), serum TC, TG and LDL levels were found to be significantly (P<0.05) elevated whereas HDL levels decreased when comparing to C group. This hyperlipidemia occurs due to increased breakdown of lipid and free fatty acids from peripheral stores. Moreover, hyperlipidemia with elevated levels of triglycerides results in oxidative stress and inflammation which may independently potentiate the adverse effects of hyperglycemia [33]. In the present study the increased levels of blood glucose, TC, TG and LDL in STZ induced rats (DC) were significantly (P<0.05) lowered by the administration of DFP as compared to DC group. Moreover, DFP significantly (P<0.05) increases the level of serum HDL-c when compared to DC group, which is in the line with the findings of Kaur et al. [33] and Kaithwas and Majumdar [34]. While these results disagree with the findings reported by Paschos et al. [35] who found that flaxseed powder and flaxseed oil have the ability to lower blood cholesterol levels in vitro and in vivo studies on animal models.
Antihyperglycemic effect of flaxseeds powder can be attributed to the presence of Secoisolariciresinol diglucoside (SDG) [36]. SDG is the most abundant lignan in flaxseed. The lignan SDG is present in a greater quantity in the seed coat. In humans, ingested SDG is converted by bacteria in the colon to biologically active lignans enterodiol and enterolactone [37]. These SDG metabolites possess antioxidant activity and have been shown effectively inhibit the development of type 1 and 2 diabetes. On the other hand, such an effect of flaxseeds could be related to the partial regeneration or preservation of pancreatic β-cell mass after STZ treatment [38].
The antihyperlipidemic effect of flaxseed is attributed to flaxseed and its derivative flaxseed oil which are known rich sources of unsaturated fatty acids such as oleic acid, and linoleic acid, which in return play a crucial role in reducing blood cholesterol in both humans and rats [39]. Fixed oil as a precursor to omega-3 fatty acids also improve VLDL turnover. VLDL is converted to VLDL remnants, which may be rapidly cleared by LDL receptors in the liver or further metabolized to LDL. The antihyperlipidemic activity of fixed oil could possibly be attributed to, (i) increase in hepatic LDL receptor activity, (ii) increasing the clearance of LDL from plasma and acceleration of the uptake of VLDL remnants by the liver, allowing fewer of these particles to be converted to LDL and thereby reducing the rate of LDL formation and (iii) inhibition of HMG-COA reductase and subsequent reduction in production of TG and VLDL in the liver [40].
Interestingly, Movassat and Portha [41] recently found that conjugated linoleic acid and Omega-3 fatty acids can activate keratinocyte growth factor (KGF) expression. KGF can improve β-cell regeneration in rats with streptozotocin-induced diabetes. Thus, flaxseed supplementation could probably lead to increase KGF expression, thus contributing to pancreatic regeneration and/or preservation. Similar data were found in vivo in mice in which the dietary supplementation of conjugated linoleic acid and Omega-3 polyunsaturated fatty acids augmented insulin secretion partly because of increased islet glucose oxidation [42,43]. Thus, the supplementation of diet with flaxseed which provided linoleic and linolenic acid had a protective or regenerative effect on the endocrine pancreas.
The most important result of the present study was that diabetic rats, fed on 10% flaxseed powder diet (DFP) were able to partly recover from STZ-induced diabetes within 8 weeks compared to DC.
Conclusion
It can be concluded from the results that the administration of flaxseed powder has better antihyperglycemic and antihyperlipidemic effects. The study also suggests the responses in blood parameters in these animals are also demonstrated that flaxseed powder supplementation may act as both antioxidant agents as well as hypoglycemic agents. Moreover, flaxseed powder can be an excellent adjuvant support in the curative of diabetes mellitus and its complications in STZ induced diabetic rats.
References
- Aloufi BH (2018) The hypoglycemic effect of flaxseed oil in alloxan-induced diabetic rats. Adv Biores 9: 155-159.
- Abolfathi AA, Mohajeri D, Rezaie A, Nazeri M (2012) Protective effects of green tea extract against hepatic tissue injury in streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med 2012: 740671.
- Gali-Muhtasib H, Roessner A, Schneider-Stock R (2006) Thymoquinone: a promising anti-cancer drug from natural sources. Int J Biochem Cell Biol 38: 1249-1253.
- Sun B, Cheng X, Lc M, Tian H, Cl L (2014) Relationship between metabolic diseases and all-cause and cardiovascular disease death in elderly male diabetics during a 10-year follow-up. Zhonghua Yi Xue Za Zhi 94: 591-595.
- Fan D, Li L, Li Z, Zhang Y, Ma X, et al. (2018) Effect of hyperlipidemia on the incidence of cardio-cerebrovascular events in patients with type 2 diabetes. Lipids Health Dis 17: 102.
- Yari Z, Rahimlou M, Poustchi H, Hekmatdoost A (2016) Flaxseed supplementation in metabolic syndrome management: a pilot randomized, open-labeled, controlled study. Phytother Res 30: 1339-1344.
- Kaithwas G, Majumdar DK (2010) Evaluation of antiulcer and antisecretory potential of L. usitatissimum (Linseed/Flaxseed) fixed oil and possible mechanism of action. Inflammopharmacology 18: 137-145.
- Izzo AA, Hoon-Kim S, Radhakrishnan R, Williamson EM (2016) A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytother Res. 30: 691-700.
- Shim YY, Gui B, Arnison PG, Wang Y, Reaney MJT (2014) Flaxseed (Linum usitatissimum L.) bioactive compounds and peptide nomenclature: a review. Trends Food Sci Tech 38: 5-20.
- Fukumitsu S, Aida K, Ueno N, Ozawa S, Takahashi Y, et al. (2008) Flax seed lignan attenuates high-fat diet-induced fat accumulation and induces adiponectin expression in mice. Br J Nutr 100: 669-676.
- Dahl WJ, Lockert EA, Cammer AL, Whiting SJ (2005) Effects of flax fiber on laxation and glycemic response in healthy volunteers. J Med Food 8: 508-511.
- Al-Ani I, Abired AN, Mustafa BE, Abdel Wahab EN, Azzubaidi MS (2017) Effect of flaxseed extract on the liver histological structure in streptozotocin induced diabetic rats. Int Med J Malaysia. 16: 91-98.
- Barre DE, Mizier-Barre KA, Griscti O, Hafez K (2008) High dose flaxseed oil supplementation may affect fasting blood serum glucose management in human type 2 diabetics. J Oleo Sci 57: 269e273.
- Campbell AP (2003) Flax facts. A grain for good health. Diabetes Self Manag 20: 18, 20-22.
- Reeves PG, Nielsen FH, Fahey GC (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123: 1939-1951.
- Chapman DG, Castillo R, Campbell JA (1959) Evaluation of protein in foods. I. A method for the determination of protein efficiency ratios. Can J Biochem Physiol 37: 679-686.
- Chen P, Zhang Q, Dang H, Liu X, Tian F, et al. (2014) Antidiabetic effect of Lactobacillus casei CCFM0412 on mice with type 2 diabetes induced by a high-fat diet and streptozotocin. Nutrition 30: 1061-1068.
- Wright JR, Fraser RB, Kapoor S, Cook HW (1995) Essential fatty acid deficiency prevents multiple low-dose streptozotocin induced diabetes in naive and cyclosporinetreated low-responder murine strains. Acta Diabetol 32: 125-130.
- Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 25: 192-205.
- Claiborne A (1985) Catalase activity in: Greenwald RA, CRC Handbook of Methods for Oxygen Radical Research, CRC Press, Boca Raton, Florida, USA.
- Young DS (1995) Effect of drugs on clinical laboratory tests. Ann Clin Biochem 34: 579-581.
- Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of concentration of LDL-cholesterol in plasma, without use of preparative ultracentrifuge. Clinical Chem 18: 499-502.
- Kroliczewska B, Mista D, Ziarnik A, Zuk M, Szopa J, et al. (2018) The effects of seed from Linum usitatissimum cultivar with increased phenylpropanoid compounds and hydrolysable tannin in a high cholesterolfed rabbit. Lipids Health Dis 17: 76.
- Sun J, Tang Y, Yu X, Xu Y, Liu P, et al. (2016) Flaxseed lignans alleviate high fat diet-induced hepatic steatosis and insulin resistance in mice:Potential involvement of AMP-activated protein kinase. J Funct Foods 24: 482-491.
- Wang H, Wang J, Qiu C, Ye Y, Guo X, et al. (2017) Comparison of phytochemical profiles and health benefits in fiber and oil flaxseeds (Linum usitatissimum L.). Food Chem 214: 227-233.
- Swanston-Fiatt SK, Day C, Bailey CJ, Flatt PR (1990) Traditional plant treatments for diabetes: studies in normal and streptozotocin diabetic mice. Diabetologia 33: 462-464.
- Nogueira MS, Kessuane MC, Lobo Ladd AA, Lobo Ladd FV, Cogliati B, et al. (2016) Effect of long-term ingestion of weakly oxidised flaxseed oil on biomarkers of oxidative stress in LDL-receptor knockout mice. Br J Nutr 116: 258-269.
- Amresh G, Kant R, Zeashan H, Gupta RJ, Rao ChV, et al. (2007) Gastroprotective effects of ethanolic extract from Cissampelos pareira in experimental animals. J Nat Med 61: 323-328.
- Hewawasam RP, Jayatilaka KAPW, Pathirana C, Mudduwa LKB (2003) Protective effect of Asteracantha longifolia extracts mouse liver injury induced by carbon tetrachloride and paracetamol. J Pharm Pharmacol 55: 1413-1418.
- Hussein J, Latif YA, Medhat D, El-Bana M, El-Khayat Z (2017) Evaluation of Asymmetric Dimethylarginine in Diabetic Rats Treated with Flaxseed Oil. Int J Pharms and Nutrl Sci 1: 1-5.
- Pilar B, Gullich A, Oliveira P, Stroher D, Piccoli J, et al. (2017). Protective Role of Flaxseed Oil and Flaxseed Lignan Secoisolariciresinol Diglucoside Against Oxidative Stress in Rats with Metabolic Syndrome. J Food Sci 82: 3029-3036.
- Han HF, Qiu H, Zhao H, Tang X, Li D, et al. (2018) Dietary flaxseed oil improved western-type diet-induced atherosclerosis in apolipoprotein-E knockout mice. Journal of Functional Foods 40: 417-425.
- Kaur N, Kishore L, Singh R (2017) Therapeutic effect of Linum usitatissimum L. in STZ-nicotinamide induced diabetic nephropathy via inhibition of AGE’s and oxidative stress. J Food Sci Technol 54: 408-421.
- Kaithwas G, Majumdar DK (2012) In vitro antioxidant and in vivo antidiabetic, antihyperlipidemic activity of linseed oil against streptozotocin-induced toxicity in albino rats. Eur. J Lipid Sci Technol 114: 1237-1245.
- Paschos GK, Magkos F, Panagiotakos DB, Votteas V, Zampelas A (2007) Dietary supplementation with flaxseed oil lowers blood pressure in dyslipidaemic patients. Eur J Clin Nutr 61: 1201-1206.
- Prasad K (2008) Regression of hypercholesterolemic atherosclerosis in rabbits by secoisolariciresinol diglucoside isolated from flaxseed. Atherosclerosis 197: 34-42.
- Hano C, Martin I, Fliniaux O, Legrand B, Gutierrez L, et al. (2006) Pinoresinol-lariciresinol reductase gene expression and secoisolariciresinol diglucoside accumulation in developing flax (Linum usitatissimum) seeds. Planta 224: 1291-1301.
- Prasad K (2000) Antioxidant activity of secoisolariciresinol diglucosidederived metabolites, secoisolariciresinol, enterodiol, and enterolactone. Int J Angiol 9: 220-225.
- Makni M, Fetoui H, Gargouri NK, Garoui el M, Zeghal N (2011) Antidiabetic effect of flax and pumpkin seed mixture powder: effect on hyperlipidemia and antioxidant status in alloxan diabetic rats. J Diabetes Complications 25: 339-345.
- Kaithwas G, Majumdar DK (2012) In vitro antioxidant and in vivo antidiabetic, antihyperlipidemic activity of linseed oil against streptozotocin-induced toxicity in albino rats. Eur. J Lipid Sci Technol 114: 1237-1245.
- Movassat J, Portha B (2007) Early administration of keratinocyte growth factor improves beta-cell regeneration in rat with streptozotocin-induced diabetes. J Endocrinol 195: 333-340.
- Winzell MS, Pacini G, Ahren B (2006) Insulin secretion after dietary supplementation with conjugated linoleic acids and n-3 polyunsaturated fattyacids in normal and insulin-resistant mice. Am J Physiol Endocrinol Metab 290: 347-354.
- Hewawasam RP, Jayatilaka KAPW, Pathirana C, Mudduwa LKB (2003) Protective effect of Asteracantha longifolia extracts mouse liver injury induced by carbon tetrachloride and paracetamol. J Pharm Pharmacol 55: 1413-1418.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi