Research Article, J Nucl Ene Sci Power Generat Technol Vol: 12 Issue: 1
Thermodynamic Principles for Removing Tritium from Treated Water in Fukushima Daiichi by Thermal Membrane Processes
Malik Fakron*
Department of Mechanical Engineering, Benghazi University, Benghazi, Libya
*Corresponding Author: Malik Fakron
Department of Mechanical Engineering, Benghazi University, Benghazi, Libya
E-mail: malikfakron@protonmail.ch
Received: 08-Dec-2022, Manuscript No. JNPGT-22-82757;
Editor assigned: 12-Dec-2022, PreQC No. JNPGT-22-82757 (PQ);
Reviewed: 26-Dec-2022, QCNo JNPGT-22-82757;
Revised: 02-Dec-2022, Manuscript No. JNPGT-22-82757 (R);
Published: 12-Jan-2022 DOI: 10.35248/2325-9809.22.11.1000306.
Citation: Fakron M (2022) Thermodynamic Principles for Removing Tritium from Treated Water in Fukushima Daiichi by Thermal Membrane Processes. J Nucl Ene Sci Power Generat Technol 12:1.
Abstract
This research paper provides theoretical scientific evidence for removing tritium from ALPS treated water in fukushima daiichi. This research is a theoretical study about the possibility of using a membrane thermal separation process for removing tritium from ALPS treated water in fukushima daiichi power plant. The negative effects of the presence of tritium in natural water are the main reason for this research on separation of tritium from nuclear power plant water. In previous research studies there are references to separation of tritium by a thermal membrane process. This pervaporation process based on experimental research. This paper describes thermodynamics based on the separation of tritium from ALPS treated water as scientific evidence
Keywords: Tritium; Thermodynamics; ALPS treated water; Thermal membrane; Nuclear power plant water
Abbreviations
R: Universal Gas Constant; PVAPOUR PRESSURE: Vapour Pressure for HTO or Light Water in Mixture; PHTO: Vapour Pressure for Pure HTO; PH2O Pressure for Pure Light Water; PVACCUM PRESSURE: Vacuum Pressure on Permeate Membrane Side; T: Temperature K; VHTO: Saturation Vapour Partial Molar Volume HTO; VH2O : Saturation Vapour Partial Molar Volume Light Water; XHTO : Mole Fraction for HTO; XH2O: Mole Fraction for Light Water; μHTO: Chemical Potential HTO in Mixture; μH2O : Chemical Potential of Light Water in Mixture; PV: Pre Vapour.
Introduction
Following a major earthquake, a powerful tsunami disabled the power supply to nuclear reactors in Fukushima Daiichi nuclear power plant. On Friday 11 March 2011, an accident contaminated the soil and the water environment in a large area in fukushima prefecture. The main contaminants are radioactive caesium and tritium in water. The main reason for this paper is to provide thermodynamics principles for tritium separation from ALPS treated water. Tritium is a radioactive hydrogen isotope with a half-life of 12.5 years that is emitted by a nuclear reactor cooling system. More than 500 large reactors exist around the world. Tritium is produced during the operation of nuclear reactors of all types. Tritium is present in many organic compounds, including those which are biologically important. Tritium is associated with radiological risk if the human body absorbs it in organic molecules throughout the environment along food chains to its potential human consumer. Inorganic tritium is bonded to the carbon skeleton of organic molecules. In this case, non-exchangeable tritium is formed in aquatic life. The interaction of tritium with aquatic life, plants, or fish causes tritium accumulation and retention in biological structures, then in the food chain, and finally in the human body.
The chemical potential of organisms in thermodynamics is the energy that can be absorbed or removed due to a change in analyte concentration of the specific organism, such as a nuclear reaction or phase separation. The chemical potential of organisms in a combination is described as the rate of change of a thermodynamic system's free energy with respect to the number of atoms or molecules of the organisms included in the system. As a result, it is the partial derivative of the free energy with respect to the amount of the organisms, with the concentration levels of the other organisms in the combination remains constant. The chemical potential is the partial molar Gibbs free energy when both temperature and pressure are held constant and the amount of particles is expressed in moles.
Materials and Methods
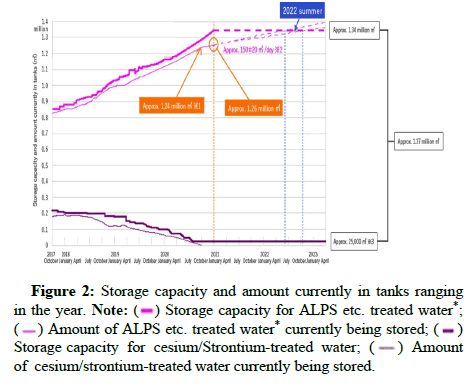
Tritium in the human body is replaced by the hydrogen in DNA molecules and increases cancer genic risk due to radiation effects on the human body [1-3]. These negative effects of the presence of tritium in natural water are the main reason for the separation of tritium from nuclear power plant water. The complete process for treatment contaminated water and quantity contaminated water in Fukushima daiichi plant as shown in Figures 1-3.
Figure 2: Storage capacity and amount currently in tanks ranging
in the year.
Note: ( ) Storage capacity for ALPS etc. treated water*;
(
) Storage capacity for ALPS etc. treated water*;
( ) Amount of ALPS etc. treated water* currently being stored; (
) Amount of ALPS etc. treated water* currently being stored; ( )
Storage capacity for cesium/Strontium-treated water; (
)
Storage capacity for cesium/Strontium-treated water; ( ) Amount
of cesium/strontium-treated water currently being stored.
) Amount
of cesium/strontium-treated water currently being stored.
Theory and Formula
Theoretical design for any process is evaluated through the difference in chemical potential between first state and second state. The second state has lower chemical potential for the process. Any system has tendency to moving in more stable state. The stable state has lower molar Gibbs free energy and more entropy [4].
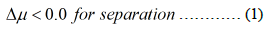
The process is based on generating chemical potential difference less than zero for required compound to be separated from liquid mixture on membrane side and vacuum side of membrane and other compounds have chemical potential greater than zero. In fukushima daiichi ALPS treated water; there is mixture of HTO and light water. The main processes for separating HTO from light water are used pervaporation and vacuum membrane distillations [5]. The vapour pressure for HTO 35.57097 Kpa at 75.6°C and the light water vapour pressure is 39.503423 Kpa. This process is used vacuum pressure at vacuum membrane side is 37 Kpa and heating the liquid mixture at 76°C before entering membrane compartment in liquid side [6,7]. This separating light water from mixture by evaporation and concentrating HTO in liquid phase and enrichment HTO in mixture. Vacuum Membrane Distillations (VMD) is amongst the most favourable MD configurations. In this process, the vapour is withdrawn by exerting a vacuum pressure to the permeate side of the membrane, which is kept as just lower than the saturation pressure of volatile components in the hot feed.
The Per-Vaporation (PV) is a membrane separation process for separating liquid mixtures wherein the upstream of dense PV membranes is in contact with feed liquids and the downstream is kept at vacuum state. These two processes vacuum membrane distillations and pervaporation are same principles, but the difference is membrane type. The chemical potential of tritiated water HTO and light water in the feed and the vapour at vacuum side, the difference in chemical potential of HTO and light water in feed side and vacuum side is given by [8-10].
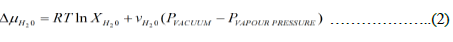
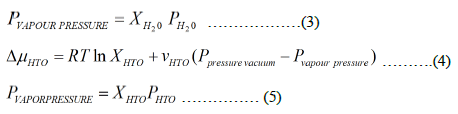
The isothermal condition, the chemical potential difference (J/mol) between the feed side and vacuum side is given by assuming that the activity coefficient is unity and the partial molar specific volume of HTO and light water.
Results and Discussions
Thermodynamics principles for any chemical or physical process, this thermodynamic base is explained main process parameters in symbolic logic form, the molar Gibbs free energy or chemical potential function is explained for any process from one state to second [11-15]. The system is moved in direction of increasing entropy and decreasing Gibbs free energy. To overcome this, applying another potential such as vacuum pressure in second state or any other potential as in any separation process. These equations from 1 to 5 are the main design process equations and main parameters. Considering these equations for design separation thermal membrane process (membrane distillations and pervaporation) as in the ALPS treated water in fukushima daiichi [16-19]. Nuclear power plant. The Fukushima Daiichi. Nuclear waste effluent treatment is the largest ever nuclear waste treatment process [20-22]. The only remaining problem is tritium removing from ALPS treated water in Fukushima Daiichi. Nuclear power plant. For using membrane distillations process and pervaporation process. The only adjusting process parameters for removing tritium from light water. The main parameters for using membrane distillations process and pervaporation process are tritium mole fraction and light water mole fraction and process temperature and membrane type for pervaporation and vacuum pressure on permeate side on membrane to enrichment tritium in process feed side. Pilot study also providing more and details information for increasing process performance. The mole fractions for tritium and light water play important role based on the value of mole fraction in first term in equations 4 and 2. Providing numerical value for the possibility for removing tritium from mixture or no. This thermodynamics principle are played crucial role in design any process for any purpose.
Conclusion
Tritium is harmful for aquatic life; Tritium is associated with radiological risk if the human body absorbs it in organic moleculesthroughout the environment along food chains to its potential human consumer.
Thermal membrane process, Membrane distillations and pervaporation are abled for separation tritium from light water but the adjustment process parameters and thermodynamic analysis are very important for reaching separation tritium from light water.
Pervaporation membrane process was used in separation tritium in many previous experimental research studies without any thermodynamic analysis.
Thermal membrane process such as membrane distillations and pervaporation are very useful, more economic and not sophisticated, suitable for removing tritium from ALPS treated water in Fukushima Daiichi. Nuclear power plant.
The equations are derived for mixture of light water and tritium hydroxide HTO due to lack for details analysis for ALPS treated water.
In case the results of equations 2 and 4 negative, which meaning the tritium and light water will cross membrane in permeate side. Use membrane distillations with sweeping gas membrane distillations and use very low pressure in gas side less than vapour pressure of light water and higher than tritium heavy water HTO and add to equation chemical potential function mole fraction term in permeate side.
References
- Ondareva L, Kudryasheva N, Tananaev I (2022) Tritium: Doses and Responses of Aquatic Living Organisms (Model Experiments). envir 9(4):51.
- Bell AC, Perevenzentsev AN (2006) Method and apparatus for concentrating tritiated water. European Patent Appl EP 1736439A2.
- Belovodsky LF, Gayevoy VK, Grishmanovsky VI (1985) Tritium; Energoatomizdat: Moscow, Russia p.212.
- Balzhiser RE, Samuels MR, Samuels M, Eliassen JD (1972) Eliassen Chemical Engineering Thermodynamics; the Study of Energy, Entropy, and Equilibrium.
- Embury MC, Kent LR, Erwin MG (1986) Tritium recovery by cryogenic hydrogen isotope distillation. Proceedings of AIChE winter annual meeting Miami 2-7.
- Frilette VJ, Hanle J, Mark H (1948) Rate of exchange of cellulose with heavy water. J Am Chem Soc 70:1107–1113.
- Galeriu D, Melintescu A (2010) Tritium in Radionuclides in the Environment. West Sussex: John Wiley& Sons pp. 47–64.
- Chae JS, Kim G (2018) Dispersion and removal characteristics of tritium originated from nuclear power plants in the atmosphere. J Environ Radioact 192:524–531.
[Crossref] [Google Scholar] [Pub Med]
- Habibi Y, Lucia LA, Rojas OJ (2010) Cellulose nano-crystals: Chemistry, self-assembly, and applications. Chem Rev 110:3479-3500.
[Crossref] [Google Scholar] [Pub Med]
- Cox JD (1982) Notation for states and processes, significance of the word standard in chemical thermodynamics, and remarks on commonly tabulated forms of thermodynamic functions. Pure Appl Chem 54(2):1239-1250.
- Holtslander WJ, Tsyplenkov V (1984) Management of tritium at nuclear facilities. In Radioactive waste management.
- Quisenberry DR (1979) Environmental aspects of tritium. Environ Pollut 20:33-43.
- Rubel M (2019) Fusion Neutrons: Tritium Breeding and Impact on Wall Materia12ls and Components of Diagnostic Systems. J Fusion Energ 38:315–329.
- Mikhailova VN (1997) Nuclear Tests of the USSR; Russian Federal Nuclear Center-All- Russian Research Institute of Experimental Physics. Serov, Russia p.302.
- Weinberg AM (1981) The future of nuclear energy. Phys Today 34(3):48–56.
[Crossref]
- Garbinsky L, Psenicka L (1979) Vyuzitie monitorov rádioaktivity v zivotnom prostredi. InZb. 4-Vcd. Konf. SVST: Tvorba a jchr. Zivot. Prostred., Bratislava 1: p.260.
- Pilmer DF, Denovan JT (1973) Comprasion of predicted and measured radoinuclide concentrations in marine animals near an operating PWR. Trans Am Nucl Soc 17:29–30.
- Yamanishi T, Kakiuchi H, Tauchi H, Yamamoto T, Yamamoto I (2020) Discussions on Tritiated Water Treatment for Fukushima Daiichi Nuclear Power Station. Fusion Sci Technol 76(4):430- 438.
- Dolan TJ, Longhurst GR, Garcia-Otero E (1992) A Vacuum Disengager for Tritium Removal from HYLIFE-II Reactor Flibe. Fusion Technol 21(3P2B):1949-1954.
- (2020) Plan for the Stabilization and Treatment of Waste generated by Contaminated Water Purification and Treatment Facilities.
- Moir RW, Lee JD, Fulton FJ, Huegel F, Neef Jr WS. et al., (1985) Design of a Helium-Cooled Molten-Salt Fusion Breeder. Fusion Technol 8, 1(Part 2A):465.
[Crossref] [Google Scholar]
- Wee SL (2008) Membrane separation process-pervaporation through zeolite membrane. Sep Purif Technol 63:500-516.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 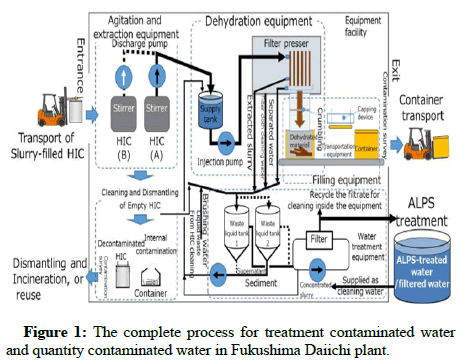
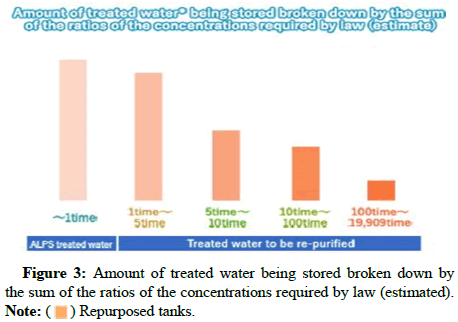
 ) Repurposed tanks.
) Repurposed tanks.

