Review Article, J Nucl Ene Sci Power Generat Technol Vol: 9 Issue: 3
Toxicity and Remediation of Radioactive Pollutants
Hosam M Saleh* and Samir B Eskander
Radioisotope Department, Nuclear Research Center, Atomic Energy Authority, Dokki, Giza, Egypt
*Corresponding Author : Hosam M Saleh
Radioisotope Department, Nuclear Research Center, Atomic Energy Authority, Dokki, Giza, Egypt
Tel: +201005191018
Fax: +20237493042
E-mail: hosam.saleh@eaea.org.eg; hosamsaleh70@yahoo.com
Received: July 29, 2020 Accepted: August 05, 2020 Published: August 10, 2020
Citation: Saleh HM, Eskander SB (2020) Toxicity and Remediation of Radioactive Pollutants. J Nucl Ene Sci Power Generat Technol 9:3. doi: 10.37532/jnpgt.2020.9(3).197
Abstract
Radioactive wastes considered the most threats to the present and coming humankind, simply because of its widely spreading, the longlived of most radioactive pollutants, and its severity toxic impacts. The present chapter details the radioactive wastes definition and their categories, in addition to their sources and risks. Radiation exposure is regulated on the assumption that any exposure carries some risk of a health effect. Evaluation of radioactive pollutants toxicity, indefinitely, is a complicated and hard task and depends, mainly, on the radiotoxicity of radioisotope contented in the generated wastes. The nuclear and radioactive waste hierarchy works based on well-established disposed safety standards rules for their management. Those rules and guidelines are progressed by international and national organisations and recommended under a framework of co-operative plans to aid countries to develop and sustain national safety standards. Insignificant, improper and illegal management of the hazardous radioactive wastes affect seriously human health and his surrounding ecosystems and put human being under the threats of infections, toxic impacts and hurts besides the extensive environmental damages. The candidate standards put forward to ensure the safety of the citizens and his environment, both now and for the future. Therefore, it is recommended that more researches, studies and efforts have to be undertaken through the United Nations to raise awareness of the radioactive hazard wastes problems, encouraging national and international cooperative action plans, and to find the solutions, especially, in the low-income countries.
Keywords: Radioactive wastes; Toxicity; Social impacts; Management
Introduction
All the distinguish man facilities will come to nothing if world future generations are diseased, handicapped, and deaden on a gigantic scale due to toxic by-products of the present generation's technological achievements. Radioactive pollution considered the most threats to the present and coming humankind, simply because of its widely spreading, the long-lived radioactive pollutants, and its severity toxic effects.
Radioactive Pollution can be defined as the abrupt elevation in the natural background radiation levels due to un-controlled activities in various nuclear fields and accompanied by the release of radioactive pollutants to the surrounded ecosystem. These activities include mining and milling, production, handling and processing of radioactive materials, transportation, storage and final disposal of radioactive wastes, as well as the nuclear power plants for research and energy production, along with the use of radionuclides in medicine and research. It is estimated that more than 20% of radiation we are exposed to is due to those activities. Therefore, it is very important to differentiate between background radiation and the radiation which emanated due to radioactive pollutants.
Although it is out of the scope of this chapter, we have to ask, also, what about the other sources of radiation e.g. microwaves, cell phones, radio transmitters, wireless devices, computers, and other common commodities of today’s life?
According to Posudin Y, the radioactive pollution is defined as the physical pollution of living organisms and their environment (atmosphere, hydrosphere, and lithosphere) as a result of the release of radioactive substances into the environment during nuclear explosions and testing of nuclear weapons, nuclear weapon production and decommissioning, mining of radioactive ores, handling and disposal of radioactive waste, and accidents at nuclear power plants [1]. It is worth to state that, the northern hemisphere is much more contaminated with radioactive pollutants than the south. The reason behind this is referred to that 90% of all nuclear weapons tested was done in the northern part, and the most known nuclear power-plant disasters all occurred in the northern hemisphere e.g. Three Miles Island, Chernobyl and Fukushima Daiichi nuclear disasters [2] (Figure 1).
Figure 1: Fukushima Daiichi nuclear disaster: Fukushima damaged reactors. In this photograph, smoke escapes from the roof of reactor No. 3 which has just been blown by an explosion, forty hours after the one that happened on unit 1. The explosion that was to occur on reactor No. 2 has yet to come. These non-explosions, but hydrogen explosions due to large amounts of hydrogen produced by the prolonged absence of cooling, and that leaked and mixed with air. Source: Japan Atomic Industrial Forum, Tepco.
The contamination of the environment with radioactive pollutants represents a serious health risk to man and other living organisms. Radioactive pollutants differ from other conventional pollutants in that it cannot be detoxified or broken down into harmless materials, but they must be isolated from the environment until their radiation level decreased to the safe level, a process which can persist thousands of years for some radioactive pollutants.
The main aim of this paper is to provide advances and perspective on the Radioactive Pollutants: Occurrence, Analysis, Toxicity and Remediation. It includes descriptions of studies, investigations and provides conclusions, where possible, on the relevance of toxicity to public health.
Types of Radioactive Pollution
Based on the frequency with which the radioactive pollution happens, it can be continuous, occasional or accidental.
Continuous pollution
It is the type of pollution constantly generated from uranium mines, nuclear reactors, and test laboratories, where the radioactive contaminants are always present.
Occasional pollution
It is the type of pollution that occurs during nuclear tests or experimental tests on radioactive substances.
Accidental pollution
It is the type of pollution that occurs when certain activity involving dangerous radioactive material fail and it gets out of control.
The Occurrence of Radioactive Pollutants
Radioactive pollution is a consequence of the release of radioactive pollutants into the air, water, or earth as a result of man nuclear activity, either by accident or by design. Radioactive pollution according to Hub Pages report, like any other kind of pollution is the discharge of something unwanted into the environment.
There are two broad sources of radioactive pollution: Natural sources and anthropogenic sources.
Natural sources
Small amounts of radioactive materials are contained in mineral springs, sand mounds and volcanic eruptions. Essentially all substances contain radioactive elements of natural origin to some extent or the other [3].
Artificial sources
The human activity have also added radioactivity artificially to the natural one. Two main sources have been: (a) the civilian nuclear programmes, including nuclear power production, medical and industrial applications of radioactive nuclides for peaceful purposes, and (b) the military nuclear programme, including atmospheric and underground nuclear-weapon testing and weapon production [3].
Environmental encyclopaedia puts the sources of such wastes as:
1. Nuclear weapon testing or detonation
2. The nuclear fuel cycle, including the mining, separation, and production of nuclear materials for use in nuclear power plants or nuclear bombs
3. Accidental release of radioactive material from nuclear power plants. Sometimes natural sources of radioactivity, such as radon gas emitted from beneath the ground, are considered pollutants when they become a threat to human health
The causes of radioactive pollution as outlined by Hub Pages are:
• Production or testing of nuclear weapons: Radioactive materials used in this production has high health risks and releases a small amount of pollution. According to Hub Pages, this release is not significant and is not a danger to us unless an accident occurs. According to IAEA, the largest source of global radiological contamination was atmospheric testing of more than 500 nuclear weapons at Semipalatinsk in Kazakhstan, on Novaya Zemlya in Russian Federation, at the Nevada test site in the USA, in the Marshall Islands, on Lop. Nor is China, on the Atolls of Mururoa and Fangataufa used by the French and on the Maralinga and Emu sites in Australia (used by the UK)
• Decommissioning of nuclear weapons: The decommissioning of nuclear weapons causes slightly more radioactive pollution than in the production, however, the waste (alpha particles) is still of low risk and not dangerous unless ingested as mentioned in Hub Pages (Figure 2). According to IAEA, the fjords of Novaya Zemlya, Russian Federation were used as dumping sites for radioactive wastes. Three reactors with spent fuel, five reactors without fuel, four vessels and numerous containers were dumped in the Abrosimov fjord since 1965
• Mining of radioactive ore (uranium, phosphate etc): Mining these involves crushing and processing of the radioactive ores and this generates radioactive waste which emits alpha particles. This waste is of low risk unless ingested or inhalant. According to IAEA, releases of radioactive materials into the environment occur in each part of the nuclear fuel cycle from mining and milling through fuel fabrication, reactor operation and reprocessing of spent fuel to the end of cycle operations as waste management [4]
• Coal ash: Some coal contains more radioactive material than usual and is often referred to as "dirty coal"; when this is burnt the ash becomes more radioactive. Due to small amounts being released into the atmosphere and its ability to be inhaled, this ash is significantly more dangerous
• Medical waste: radioactive isotopes are used in medicine, either for treatment or diagnostics. These can be left to decay over a short period after which they can be disposed of as normal waste as mentioned in Hub Pages
• Nuclear power plants: Accidents at the power plants can cause dangerously radioactive pollution; an example of such is in the case of Chernobyl, the most well-known and worst nuclear disaster in history, and the more recent Fukushima, after an earthquake and a tidal wave in Japan
Risk Analysis
Hazard identification
This first step in a risk assessment is the identification of the type and nature of adverse effects that an agent can cause in a population, based on studies in humans and laboratory animals. Hazard identification is the process used to identify the specific radiation sources (i.e. radionuclides) and the type of harm they could cause.
Dose-response relationship
This second step examines the relationship between exposure to a particular agent and any adverse health effects in humans as a result of this exposure. The relationship is usually based on existing evidence from epidemiological studies that describe the endpoints for adverse human health effects at relevant exposures and the dose-response relationships for the different endpoints. The endpoints considered include cancer as well as non-cancer risks.
Exposure assessment
This step gathers information about how much of a particular substance different groups have been exposed to, how the exposure took place (i.e. through which exposure pathways) and for how long the exposure occurred. Doses for the general population as well as emergency workers are considered.
Risk characterization
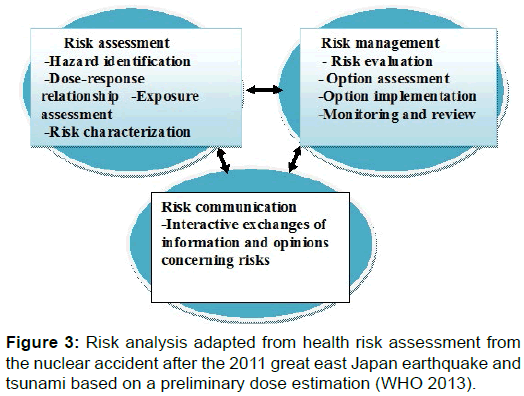
This last step of the risk assessment process integrates the information collected in the previous steps to estimate qualitatively or quantitatively the risk of adverse health effects (i.e. cancer and non-cancer risks) under defined exposure conditions. The risk characterization includes the quantitative estimation of specific cancer risks. Risk characterization takes into consideration the influence of several parameters, such as sex, age at the time of exposure, and attained age. Non-cancer risks are qualitatively assessed (Figure 3) [5].
It is very important to keep the monitoring process of food and the environment continuous and unbroken. When elevated radiation doses would be recorded, such data can be used for further improvement in the risk estimates.
Exposure Pathways
Radiation exposure is regulated on the assumption that any exposure carries some risk of a health effect. Radiation-induced health effects can be deterministic, in which biological damage is readily observed and proportional to the level of exposure, or stochastic, in which the probability of a health effect is related to the level of exposure, but the severity is not. The principal concern associated with low dose radiation exposure is the possible occurrence of cancer years after the exposure occurs [6].
The way by which ecosystem can be exposed to radioactive pollutant has nearly a primary estimate on the severity of the final impacts of the release. More than one mode of exposure can be an encounter for radioactive pollution. In some areas, direct contact is possible when the radioactive pollutant is deposited on each element of the ecosystem i.e. soil, water, air, and living organisms.
Going so far that, some portion of released radioactive pollutants would enter the water column either as a dissolved fraction or suspended aggregations, this potential pathway must not be neglected.
Some radioisotopes (radionuclides) are found as natural components of the earth’s crust. Due to technological and exploitation man's activities, those elements subjected to anthropogenic additions to soil, water and air over their natural cycles. Consequently, widespread of the radioactive pollutants are seen on a global scale and their levels are far-reaching. Ecosystems cannot burden the increased contents of these radioactive pollutants in their natural cycling and, therefore, become harmful when taken up in plants and animals at increased levels. The toxic effects of radioactive pollutants, mainly, depend on the dose released to the surrounding, the energy of the radiation emitted, how you are exposed, personal traits and habits, and the extent to which it is retained and the sites within a living organism [7].
Besides, the physical and chemical form of radionuclides, within a system is a significant determines that highly affect the surrounding environment.
The most serious exposure pathway of radioactive pollutants is due to their release from accidents or incidents took place in a nuclear facility. Post the bombs dropped on Hiroshima and Nagasaki during the second war and the atmospheric bomb tests carried out in the 1950s and 1960s, the most sounded three accidents are: the Three Mile Island (TMI) accident in 1977 in the USA, the 1986 Chernobyl disaster in the Ukrainian reactor and the last accident of Fukushima as a powerful unexpected tsunami that hit Japan in March 2011. Less articulated pollution release accidents were, also, reported during the last decades for example:
• Mayak: a military accident in 1950, which have been covered up for years by secrecy
• The 1957 graphite fire at the Windscale power plant known as the Sellafield reactor accident
• In 1999, workers at TokaïMura Japanese fuel reprocessing fa-cility violated the security regulations resulting in minor pollutants release
As a result of defective and careless control, many irradiation accidents due to the desperation of radioactive materials from medical, industrial and laboratory sources were reported. Fortunately, the consequences of these accidents' impacts were less compared to that of nuclear industry facilities. The accident in Goiania, Brazil, in 1987, is an example of criminal negligence as the exposure of a source of caesium-137 recovered by scrap merchants within a radiotherapy machine abandoned in a disused clinic. In 1984 in Mohammedia, Morocco, a source of iridium-192 used by the gamma rays diagnostics welding was picked up by a worker and had caused eight deaths (a whole family). In 1992, in Xinshou, China, a worker took home the sources of cobalt-60 for industrial use abandoned in a well (three deaths).
In 2003, in the United States, sources were found inside a truck abandoned by a bankruptcy contractor in a field. Another ruined contractor, more civic, reported by phone that he left his truck with sources inside a parking lot before crossing the Mexican border.
Generally, nuclear facilities around the world have been releasing small amounts of radioactive pollutants which have been barely detectable in comparison to the large-scale accidental releases. The same was reported by Ješkovský et al. [8].
Radioactivity from the fallout of nuclear weapons testing and nuclear power plants make up less than 0.5% of the total radiation dose, i.e., less than 0.02 millisieverts. Although the contribution to the total human radiation dose is extremely small, radioactive isotopes released during previous atmospheric testing of nuclear weapons will remain in the atmosphere for more than the next 100 years. For example, in the United States, people are exposed to about 3.50 millisieverts of ionizing radiation per year. Nearly, 82% of this radiation is due to natural sources and 18% from anthropogenic sources. The major natural source of radiation is radon gas, which accounts for about 55% of the total radiation dose.
It should be notified that the amount and type of radionuclides released during a nuclear accident are called the source term [5].
Toxicity
According to International Atomic Energy Agency (IAEA), the toxicity of a radionuclide is the ability of the nuclide to produce injury, by its emitted radiations, when incorporated in a body [9].
The hazards to human and to his environment from radioactive pollution depend on the nature of the radioactive pollutants, the concentration of pollutants, and the extent of pollution spreading. Following the IAEA, the radionuclides have been divided into three main toxicity groups, with a division of the large-medium group into two sub-groups. The choice of the dividing lines is somewhat arbitrary (Table 1).
| High toxicity |
| Pa-231, Cf-249, Th-Nat, Pu-239, Pu-240, Pu-242, Th-232, Pu-238, Ac-227, Th-230 , Np-237, Th-228, Am-241, Am-243, Cm-243, Cm-245, Cm-246, Cf-250, Cf-252 , Cm-244, U-232, Ra- 226, Ra-228, Sm- l47, U-Nat, Nd-144, U-238, Pu-241, Pb- 210, U-230, U-233, U-234, U-235, U- 236, Cm-242, Th-227, Po-210, Ra-223, Sr-90 |
| Medium toxicity |
| Upper sub-group A Ra-224, Pa-230, Bk-249 , I-129, Eu-164, Ru-106, Ce-144, Bi-210, At-211, Na-22, Co-60, Ag-110m, I-126, 1-131, Cs-134, Eu-152 (13y), Cs-137, Bi-207, Pb-212, Ac-228, In-114m, Sb - l24, Ta-182, Cl-36, Sc-46, Sb-125, Ir-192, T1-204, Ca-45, Mn-54, Y-9 1 , Zr-95, Sr-89, Cd-115m, In-115, Te-127m, Te-129m , 1-I33, Ba-140, Tb-160, Tm-170, Hf-181, Th -234 |
| Lower sub-group В P-32, V-48, Fe-59, Co-58, Ni-63, Zn-65, Rb-86, Rb-87, Tc-99, Cd-109, Sn-113,Pm-147, Sm-151, Os-185,Hg-203, As-76, Y-9 0 , Zr-97, Nb-95, Ru-103, Ag-105, Sn-125, Cs-l35, Eu-155, Gd-l53, Bi-212, K-42, As-74, Se-75, Sr-85, Nb-93m, Zr-93, Te-125m, Te-132, I-135, La-140, Tm-171, W-181, W-185, Na-24, Sc-48, Mn-52, Y-93, Tc-97m, Sb-122, Ce-141, Pr-142, Re-183, Ir-194, Bi-206, Ca-47, Co-57, Ga-72, Br-82,Cd-115, Te-131m, Cs-136, Pr-143, Ho-166, Re-188, Pa-2ЗЗ, Mo-99, Ce-l43, Dy-l66, Tc-96, Ag-111, I-132, Nd-l47, Pm-l49, Re-l36, Au-198, Tl-202, S-35, Sr-91, Os-l43, Zn-69m, As-73, As-77, Sr-92, Y-92 , Tc-97 , Pd-109, Ba-l31, Sm-153 , Eu-152 (4.2 h), Gd-159, Er-l69, W-l87, Os-l91, Ir-190, Pt-l93, Rn-220, Rn-222, Se-47, Mn-56, Ni-59, Ni-65, Kr-87, Ru- 105, Rh-105, I-134, Er-l71, Yb-175, Lu177, Re-187, Pt-l91, Pt-197, Au-196, Np-239, Si-31, Fe-55, Pd-l03, Te-l27, Au-199, Hg-l97m, TI-200, Tl-20l, Be-7, Cu-64, Hg-l97, Th-231,Nd-l49 Ru-97, In-115m, Pb-203, Cl-38, Dy-l65, Cr-51, Fl-18, C-l4, Kr-85m, Те-129, Xe-l35,Cs-l31 |
| Low toxicity |
| H-3, Zn-69, Ge-71, Nb-97, In-113m , Cs-134 m, Pt-193m, Pt-l97m, Tc-99m, Co-58m, Kr-85, Xe-133, Os-191m, Xe-131, Y-91m, Sr-85m, Tc-96m, Rh-103 |
Table 1: Radionuclides toxicity.
Adverse health effects of ionizing radiation result from two distinct mechanisms: ICPR (2007):
1. Cell killing, which can cause functional damage to the exposed tissue or organ only if a large number of cells are affected
2. Non-lethal changes in molecules of a single cell, most commonly in the DNA molecule, which can result in an increased risk of disease long after exposure [10]
Ottawa, International Commission on Radiological Protection, (Annals of the ICRP, 103(37):2-4).
There is no doubt that research on the impact of radioactive pollution, especially at low doses, will increase in the near future that can change the understanding of the risks of the radiation accident.
Evaluation of radioactive pollutants toxicity, indefinitely, is a complicated and hard task. A "released radioactive pollutant" can comprise very different kinds of materials that behave uniquely qualitatively, chemically, and decay mode. Moreover, there are numerous radioisotopes with variable half-lives, even for the same element, which can react in dramatically different ways to pollutant exposure (Table 1). Since the conditions encountered at each release exhibited a various set of physical, chemical, and biological circumstances, that delaying to provide general radioactive pollutant toxicity guidance.
As previously stated, many radioactive pollutant elements are naturally occurring in the environment, and most of them are used in anthropogenic nuclear technologies including power plants for research and electrical generation, basic components of nuclear weapons, industrial and medical applications and others. Also, the radioactive pollution of environment is a result of release of radioactive pollutants during nuclear explosions, nuclear weapon production and testing, mining of radioactive ores, decommissioning of nuclear facilities, uncontrolled handling of radioactive materials, un-proper storage and disposal of radioactive wastes, and accidents at nuclear power plants. Examples of these pollutant elements, their half-lives, their uses and their toxic effect are listed in NRC, 2000 report.
Toxicity of uranium
Elemental uranium is a dense, malleable and ductile, silverywhite metal. The most used three isotopes of natural uranium are uranium-238, uranium-234, and uranium-235. Its decay generates alpha, beta and gamma radiation.
Uses of uranium: Uranium can be used in metal or uranium dioxide form to make nuclear weapons, tank armour. Uranium -234 (t1/2 =2.46 × 105 years) is used in dental fixtures like crowns and dentures to provide natural colour and brightness. While the main usage of uranium-235 is as fuel for nuclear power plants and naval nuclear propulsion systems and used to produce fluorescent glassware, a variety of coloured glazes and wall tiles. Australia, Kazakhstan, Canada and Russia own the largest known uranium resources.
Depleted Uranium (DU) radiotoxicity: DU is a by-product of the processes for the enrichment of the naturally occurring 235U isotope. Typically, depleted uranium contains as much as 70% less 235U and as much as 80% less 234U than does naturally occurring uranium. The enrichment process reduces the radioactivity of depleted uranium to approximately half of that of natural uranium. The worldwide stockpile contains some 1½ million tons of depleted uranium. Some of it has been used to dilute weapons-grade uranium (~90% 235U) down to reactor-grade uranium (~5% 235U), and some of it has been used for heavy tank armour and the fabrication of armour-piercing bullets and missiles.
Although the chemical and the toxicological behaviours of depleted uranium are essentially the same as those of natural uranium, the respective chemical forms and isotopic compositions in which they usually occur are different. The chemical and radiological toxicity of depleted uranium can injure biological systems. Normal functioning of the kidney, liver, lung, and heart can be adversely affected by depleted uranium intoxication.
Depleted uranium can become an internal exposure hazard by inhalation, by ingestion, by subcutaneous absorption, and by dermal penetration of shrapnel or other explosion fragments. The principal sites of uranium deposition in the body are the kidneys, the liver, and the bones. Also, some material is deposited in various other tissues generally at lower concentrations than the main sites of deposition. The potential for the toxicity of uranium lies in its properties as heavy metal and as a radioactive substance. The impact of d U includes its toxicity to the Lung, renal toxicity, neurological toxicity, reproductive/ developmental toxicity, carcinogenicity, birth defects, leukaemia and soft tissue cancer [11].
It should be stated that high doses of uranium can cause serious damage to all internal organs.
Toxicity of caesium-137
Caesium-137 (t1/2=30.5 year) is an alkaline element. Due to its high water solubility, cesium-137 ions are commonly found as chemical compounds in the form of salts that easily accumulated in the living body, mainly in muscle tissue, with biological half-life10 days. Radiocesium is mainly used in moisture density measurements, liquid flow and thickness gauges, atomic clocks, in medicine for diagnosis and therapy of some types of cancers, in addition to its main uses in industry as a tracer.
Toxicity : The exposure to very high doses, in the very rare case, can lead to instant death, but low exposure to caesium-137 can result in cancer.
Toxicity of strontium radioisotopes
Strontium (Sr) is a divalent alkaline metal. It has 12 radioisotopes. Strontium-90 (t1/2=28.8 years, β-emitter), strontium-85 is radioactive with a half-life of 64.84 days, emits γ-rays and metabolized by the body in a manner close to calcium, and strontium-89 are the most famous strontium isotopes. 90Sr is commonly used in cancer radiation therapy and the fabrication of thermoelectric generators and portable power sources for space vehicles. Also, used in sensors for cigarette manufacturing. Strontium-85 is applied for scanning the calcium image in bone fractures and tumours and many industrial applications e.g. plastic, the fireworks fabrication, and paints.
All nuclear weapon tests result in large concentrations of 90Sr fallout. Nuclear disasters such as Chernobyl and Fukushima etc. together with uncontrolled industrial applications are the main sources of 90Sr release.
Toxicity of radio strontium: Radio strontium can be inhaled or ingested from the spiked ecosystem. It passes through the body, and accumulates, in extremely toxic concentrations, and remains in the bones, bone marrow and teeth causing cancer. It is worth to state that Sr-90 is one of highly toxic radionuclide, that can be absorbed into the bones of small children and large doses displace calcium in their bones causing chronic renal failure, bone deformity and tumours.
Toxicity of radium
Radium has four natural isotopes: 223Ra, 224Ra, 226Ra and 228Ra. 226Ra and 228Ra Radium is moderately soluble in water. Radium penetrates the subsurface groundwater system by the dissolution of aquifer bearing rocks, desorption from the sediment surfaces and ejection of minerals from decay series of radioactive materials in the bedrock. The predominant radium isotopes in groundwater are 226Ra and 228Ra, an alpha, beta and gamma emitters with a half-life of 1600 and 5.8 years, respectively.
Radium-223 dichloride was the first approved alpha-emitting radiopharmaceutical and is most commonly diagnosed malignancy in prostate cancer [12].
Today, luminous watch dials are painted with tritium or promethium-147 replacing radium used many years ago.
Radium is a highly radioactive “bone-seeker.” That means that when it’s ingested it makes its way to the skeleton, where it decays into other radioactive daughter elements, including radon, and bombards the surrounding tissue with alpha, beta, and gamma radiation. According to the Toxic Substances and Diseases Registry, exposure leads to “anaemia, cataracts, fractured teeth, cancer (especially bone cancer), and death.
A separate study revealed that ingestion of radium was causally associated with leukaemia in man However, further investigations are needed before a causal relationship between 226Ra in drinking water and human leukaemia can be established. Other epidemiological studies have found an increased risk of osteosarcoma and radium in drinking water. Considering the high radiotoxicity of 226Ra and 228Ra, their presence in water and the associated health risks require particular attention [13].
Toxicity of plutonium-238
Plutonium is a long-lived toxic actinide produced by neutron activation of uranium. Plutonium-238 (Pu-238) is only found in small quantities in its natural state. The main application of Pu-238 is as a heat source for radioisotope thermoelectric generators and in space exploration. Pu-238 is used as a power source for more than twenty NASA space missiles since 1972. Pu-238 has a half-life of 87.7 years and emits alpha particles as it decays.
Toxicity of Pu-238: According to IAEA classification, Pu-238 is one of the highly toxic radionuclides, and like many radioactive isotopes of heavy metals, can cause cancer if absorbed into the body through inhalation, direct contact with open wounds or ingestion of contaminated substances. The lungs, bronchia, liver and bone marrow are the most organs immediately affected by Pu-238 deposition.
Toxicity of polonium
Polonium is a rare and highly radioactive metal with no stable isotopes. Polonium has 33 known isotopes, all of which are radioactive. Po-210 is the most widely available. It is tiny traces radioisotope occurs with uranium ores. 210Po is an alpha emitter that has a halflife of 138.4 days; it decays directly to its stable 206Pb. Polonium has few applications, and those are related to its radioactivity: heaters in space probes, antistatic devices, applied for measuring the thickness of industrial coatings via attenuation of alpha radiation and sources of neutrons and alpha particles.
The target organs for polonium are the spleen and liver. 210Po is extremely dangerous; radiation exposure (both internal and external) carries a long-term risk of death from cancer.
Toxicity of chromium-51
Chromium-51 is a radioactive isotope of the chromium metal (Cr), with physical t1/2 of 27.7 days and biological half-life of 616 days. It uses in medical diagnoses for various medical parameters, especially for blood-related pathology e.g. determining Red Blood Cells (RBCs) survival time in haemolytic anaemia, determining RBCs volume or mass and assessing blood loss, lowering blood sugar levels and measuring blood flow in pregnancies. It uses also, in diagnosing the gastrointestinal protein loss.
Cr-51 emits gamma and x-ray radiations which can be fatal in large doses or with long term exposure; therefore, radio chromium must be behind lead shielding, or stored in lead containers and handled very carefully following the safety instructions.
The impact of mild exposure to Cr-51 can result in skin rashes and respiratory tract irritations, asthma, chronic rhinitis, ulceration of the nasal mucosa.
Cr-51 is toxic through both external and internal exposure. Inhalation and/or ingestion of Cr-51 leads to its deposit in the lungs, lower large intestine and kidneys, causing cancer and finally to complete dysfunctions of the suffered organs.
Toxicity of radon
Radon (Rn) A colourless tasteless, and odourless, naturally occurring radioactive, inert gas formed by radioactive decay of radium atoms in soil, and rocks. The most stable isotope is 222Rn (t1/2 =3.8 days), which is a decay product of 238U and 220Rn (t1/2 =55 sec) that occurs in the decay chain for 232Th. Therefore, the levels of Rn-222 and Rn-220 depend on the uranium and thorium contents on the soil. It uses in medicine as radiotherapy in tumours as well as an Arthritis treatment. It was reported that most of the radiation is not so much from the radon as from its decay daughter products. It was established that alpha particle emissions from inhaled radon progeny cause lung cancer which is the second leading cause of lung cancer deaths after smoking [14].
Toxicity of ruthenium-106
Ruthenium is a silver-white metallic element. Its oxides are more volatile than the metal. The most interesting oxide is RuO4. RuO4 vapours are yellow, toxic and have an odour of ozone. RuO4 can exist in significant concentration at ambient temperature. The two most important isotopes of Ru are 103Ru and 106Ru. They have half-lives of 39.6 days and nearly 1 year, respectively. Volatile RuO4 is sometimes evolved from boiling nitric acid when reactor fuels are dissolute and when fission-product wastes are concentrated [15] and/ or solidification [16].
The most important application of radioruthenium is for cancer radiation therapy, mainly, for eye and skin tumours. Also, it used in radioisotope thermoelectric generators that power satellites. Ruthenium-106 decays emitting beta particles to rhodium-106, then to stable palladium-106.
A measurable dose of radioactive ruthenium can be released, in the presence of steam and aerosol particles, as gaseous RuO4 spiking the surrounding atmosphere [17]. Ruthenium radiotoxicity is high in both short and long term and assumed to affect, mainly the respiratory tract.
Toxicity of tritium
Tritium (3H or T) is the radioisotope of hydrogen and has three times the mass of hydrogen with a physical half-life of 12.32 years. Tritium decays by emitting beta decay to the stable isotope 3He. It occurs in nature, in water and cosmic rays, or can be generated due to bombing hydrogen in a nuclear reactor [18].
Tritium is used in plastic watches, as well as in nuclear weapons and nuclear tests. It applied in researches as a radioactive tracer. It is a major tool for biomedical research, life science and drug metabolism studies. It used to produce luminous paint, and for geological prospecting and hydrology.
The harmful effect of tritium is detected when administered as tritiated water or tritiated thymidine. It has been adequately demonstrated at various levels of biological organization and can lead to the development of cancer.
Toxicity of cobalt
Cobalt (Co) is a silver, heavy metal or mineral origins. It can be used in medicine, electroplating, and pottery colouring. Co is one of the essential micronutrients for plants.
Cobalt-57 is used as a radiolabel in nuclear medicine for detecting cancerous tumours, it is also used as a component in the medical equipment as gamma cameras. On the hand, cobalt-60 is applied in medicine as cobalt radiotherapy, sterilization of medical equipment, tracing cobalt in chemical reactions, laboratory mutagenesis radiation and blood irradiation. Also, it used in the industrial field e.g. sterilization of biological-based products, products for radiography, food irradiation and production some plastic items.
Toxicity of radiocobalt: Co-57 and Co-60 are the most known radioisotopes for cobalt for their wide applications. Co-57 with a halflife 272 days and is decaying by electron capture, while Co-60 (t1/2 =5.25 years) emits two energy gamma rays. Both radionuclides are with medium radiotoxicity [19].
The exposure to high levels of Cobalt-57 damages the affected cells causing their mutation and developed cancer. In very rare cases, Co-57 may generate the Acute Radiation Syndrome, with symptoms like bleeding, diarrhoea, nausea, vomiting, coma and even sudden death. Cobalt-60 emitting relatively high energy γ rays which can cause long-lasting symptoms like fatigue and hair loss, and even loss of consciousness.
Toxicity of iodine-123
Iodine (I) is a non-metallic trace element in the form of grey crystals or violet vapours. I-123 is an artificial radioactive isotope produced in particle accelerators. It emits gamma radiation and has a half-life of only 13.22 hours. I-125 is a major diagnostic tool used in clinical tests and to diagnose thyroid disorders, also used in biomedical research. While iodine-129 is used to check some radioactivity counters in vitro diagnostic testing laboratories and I-131 is applied to treat thyroid disorders
Toxicity: Volatilization of iodine may produce an internal radiation hazard because of the tendency of iodine to be collected in the thyroid gland. Large doses of Iodine-123 are maybe toxic to the thyroid gland and surrounding tissues. It may cause hypothyroidism and other thyroid dysfunctions, including thyroid cancers.
Social and Psychological Impacts of Radioactive Pollution
It was reported that the deep impacts of nuclear accidents were often not directly due to radiation exposure, but rather social and psychological consequences [20]. Although, it is claimed that the extremely low doses of radiation that humans commonly exposed are not harmful, yet the impacts of low-level radiation are often more psychological than radiological. People exposed to low radiation dose are left in painful uncertainty about what will happen to them. Many believe they have been fundamentally contaminated for life and may refuse to have children for fear of birth defects. They may be avoided by others in their community who fear a sort of mysterious infections [21].
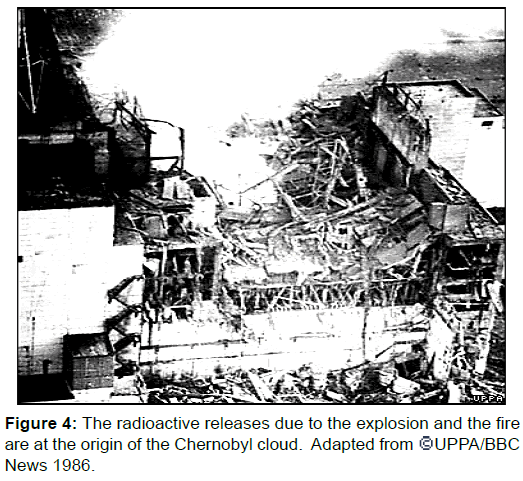
Forced evacuation from a radioactive polluted area can lead to social isolation, anxiety, depression, psychosomatic medical problems, reckless behaviour, even suicide. Such was the outcome of the 1986 Chernobyl nuclear disaster in Ukraine where more than 300,000 people were permanently evacuated from the vicinity, (Figure 4).
A comprehensive 2005 study concluded that "the mental health impact of Chernobyl is the largest public health problem unleashed by the accident to date" [21,22] commented on the 2011 Fukushima nuclear disaster, saying that "fear of ionizing radiation could have long-term psychological effects on a large portion of the population in the contaminated areas". Evacuation and long-term displacement of the suffering populations create problems for many people, especially the elderly and hospital patients [20].
Such great psychological danger does not accompany other materials that put people at risk of cancer and other deadly illness. Visceral fear is not widely aroused by, for example, the daily emissions from coal burning, although, as a National Academy of Sciences study found, this causes 10,000 premature deaths a year in the US population of 317,413,000. Medical errors leading to death in U.S. hospitals are estimated to be between 44,000 and 98,000. It is "only nuclear radiation that bears a huge psychological burden-for it carries a unique historical legacy" [21].
Catastrophic radioactive pollutions post a nuclear accident and/or production of radioactive wastes is the most important disagreements in terms of public acceptance and everlasting applications of radioisotopes in various fields of life.
The assumption that the public’s attitude to different applications of nuclear technologies is linked to the risk they gathered from the same technology, so it can broadly compare public attitudes with the consequences of a range of severe other-related accidents to allow a broad perspective on the difference between actual risk and the public understands of risk.
However, many people do not differentiate between the risks associated with peaceful applications of nuclear technologies and the risks from nuclear weapons, nuclear accidents and generated pollutions from radioactive waste disposal facilities. It is, therefore, judged that the relationship between actual and gathered risks from radioactive pollutions disclosed similarities with that benefit of peaceful applications.
Nuclear applications, for many people, seem complex technology that is difficult to control. Many have the misunderstanding that peaceful nuclear facilities can explode like nuclear weapons. As stated above, many people, also, cannot differentiate between the risks associated with peaceful applications of nuclear technologies and the risks from radioactive pollutions post any nuclear accidents.
Radioactive pollutions are considered by the majority as a critical issue to oppose nuclear peaceful applications. Many people do not differentiate between the risks associated with rewards of controlled applications and the risks from misused and/or mishandling of the technology. Support for nuclear technologies would be enhanced significantly if the matter of safe and proper management of radioactive pollution, whatever, were resolved.
Public acceptance plays an important role in the decision-making procedure for development in the radioactive pollution management and depends heavily on whether the public believes that they or their environment will be harmed by it and whether the radioisotopes applications will be risky. Considering for continued research and learning, stepped decision making, provides the opportunity to build broad societal confidence in the concept and to develop constructive relationships with the most affected regions.
Remediation of Radioactive Pollution
High doses of radioactive pollutants are spreading everywhere surrounding human ecosystems. These hazardous pollutants are present as a result of man activities or due to unexpected events. These pollutants are expanded in mining and milling of nuclear ores processing, near nuclear facilities, nuclear weapon test fields, and post-nuclear accidents. These radioactive pollutants represent an uncontrolled source of risky radiation. Therefore, proper and effective treatments of radioactive pollutants still the most important issue, from the biological and environmental concepts due to their hazardous impacts on human being health and the environment.
Strict regulations and laws are needed and should be implanted to individuals, businesses, states, government, and facilities. These regulations set standards that limit the amount of radioactive material allowed in a certain environment. These standards, also, limit, reduce and even restrict radio pollutants release and radiation doses exposure to the public from the normal applications of radioisotopes.
Source reduction
It is an elementary and the first concept to avoid elaboration and vast growing of radioactive pollutants. This can be taken through: using essential items which reduce the generation of a pollutant, using less raw virgin material when fabricating a product, multiple uses of the products, when possible, and designing products packaging to control the used quantity.
Research needs
More research on representative exposure situations concerning radioactive pollutants needs to be satisfied and systematized. Beyond the lists of well-known radioactive pollutants, there is a need to know the pollutants that exist in the environment and could have an impact on human health and his ecosystems. Furthermore, the understanding of the determinants of synergistic effects requires to be improved scientifically, putting in mind the capability to predicate synergisms in the future.
Remediation background and applications
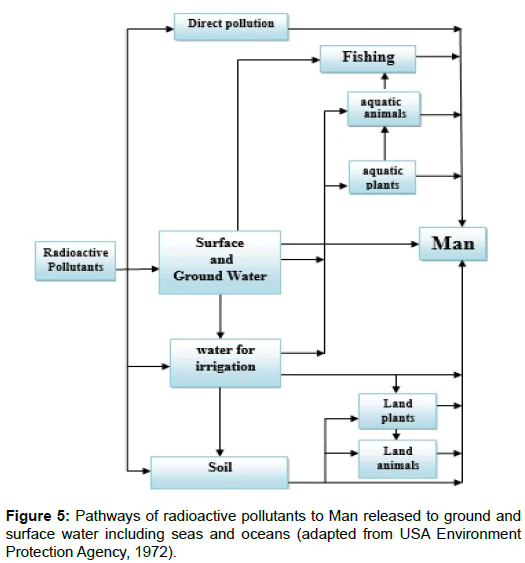
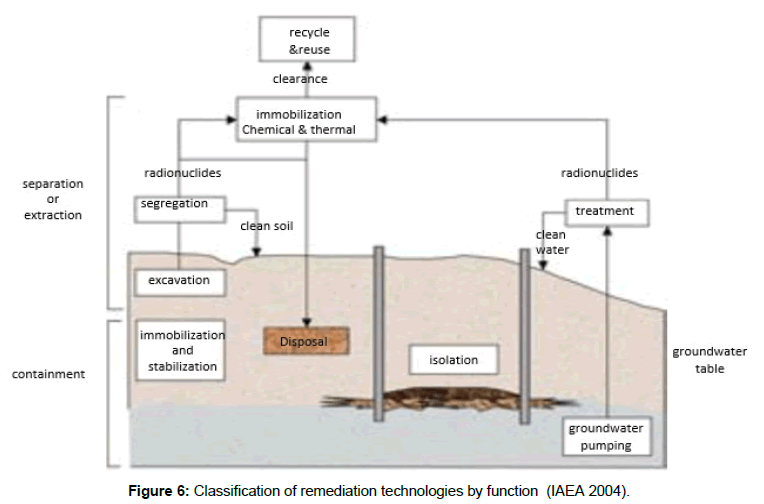
There is an array of remediation techniques exist for controlling, reducing or even removing radioactive pollutants. The most known technologies for remediating soil and/or water comprise segregation, excavation, solidification and stabilization, treatment for isolation and final disposal. The different technologies are grouped into containment and separation or extraction (Figure 5 and 6) [23].
The concentration of radiopollutant species besides its chemical state, as previously stated, are the most determining factors describing the remediation technique applied.
The main aim of immobilization is to change the radio pollutant form into one that is less susceptible to release to the surrounding ecosystem. Two basic options can be applied for immobilization depends on the position of the treatment process; in situ or ex-situ handling. In situ treatment, immobilization is carried out without the removal of polluted material i.e. in the site of the polluted item. While ex situ treatments are performed in a central plant, either inside or outside the pollution site. After treatment, the treated material is either returned or disposed of in a specially engineered repository.
The remediation operations can be categorized, based on the mode of action, into physical, chemical and biological processes which surpass finally to the concentration, stabilization and immobilization of radioactive pollutants. More than one process can be applied, for the same polluted item, to achieve the main aim of remediation.
Besides a large number of remediation techniques under investigation or at the demonstration stage, fully developed techniques are now commercially available all over the world.
Many factors are affecting the choice of certain remediation technique and include engineering and non-engineering considerations. Some of these factors are summarized as follows [1,2]:
• Radiopollutants exist and their doses, chemical and physical states
• The polluted item: air, water ... its size, configuration, location of the polluted item
• Facility including machine, materials and human resources available for the remediation process
• Effectiveness in remediating pollution
• Occupational safety and health risks associated with the technique
• Prior experience with the application of the technique
• Sustainability of any institutional control required
• Quantity and nature of secondary wastes that may be generated
• Socioeconomic considerations
• The costs associated with the remediation programme, including the temporary storage of even the final disposal
• However, there is no single technology that will be applicable in all situations and all type of radioactive pollution, yet it is worth to state that, many of the considerations for solid and liquid residual disposal can be identical, regardless of the chosen treatment technology
Several techniques, e.g. chemical precipitation, oxidationreduction, filtration, ion exchange, dialysis, and electrochemical treatments and others, are applied to remediate the radioactive pollution. Limited effectiveness, sophisticated array, higher cost, restricted application with limited prospects, and inability to improve the inherent health of the treated item make these applications used in very narrow conditions. Bioremediation process appeared as the potential alternative with eco-friendly and cost-effective remediation strategy. Bioremediation is a process of reducing radio pollutants, to limits set by regulatory agencies, based on exploiting available natural bio-resources, which mostly include plants, microbes, organic amendments, etc. that alter these contaminants. Highly porous metalorganic frameworks with excellent chemical stability and abundant functional groups have developed by Li et al. [24] and represented a new addition to the area of capturing various types of hazardous metal ion pollutants.
Treatment of radioactive polluted aqueous waste streams comprises diverse techniques such as filtration, precipitation, sorption, and/or membrane separation, [25] ion exchange [26], evaporation [27] and acid digestion and wet oxidation [28].
In the last decades newly, developed eco-friendly processes had been applied including separation and/ or bio sorption of some radio pollutants, e.g. Cs-137 and Co-60, based on agents that have a biological or natural origin [29-31].
Also, activated carbon has been applied as a potential candidate for laboratory separation process for the radioactive technetium-99 m [32].
Bioremediation is a natural process of decontaminating soil and groundwater for eliminating pollutants from the environment ineffective and eco-friendly way [33]. The different techniques of bioremediation can, also, be categorized in the two types of treatment, in situ and ex-situ [34]. In situ bioremediation approach involves treatment of polluted soils right at the point of contaminated sites itself and provides facility for avoiding excavation and transportation of hazardous radioactive pollutants; hence, there is no chance of spread of radioactivity during excavation. However, ex-situ bioremediation approach involves the excavation of pollutant soils and water from sites and subsequent transportation to a central site for the treatment [35].
Mycoremediation [36,37] is one of the biological methods for treatment of radioactive pollution and based on the usage of fungi for the removal of radiopollutant, e.g. Cs-137 and Co-60 from the ecosystem [38]. Mycoremediation relies on the efficient enzymes, produced by fungi, e.g. mushroom, for bioaccumulation, biosorption and stabilization of the pollutants [39].
Part of phytoremediation and bioremediation using terrestrial and aquatic plants for remediation of toxic elements and radionuclides based on experimental works was carried out in the Inorganic and Applied Chemistry Unit, Radioisotopes Department [40-48].
Bio concentration is a process of bioremediation concept for hazard radionuclides by utilizing some natural biological sources including bacteria, fungi, yeast, algae, etc.
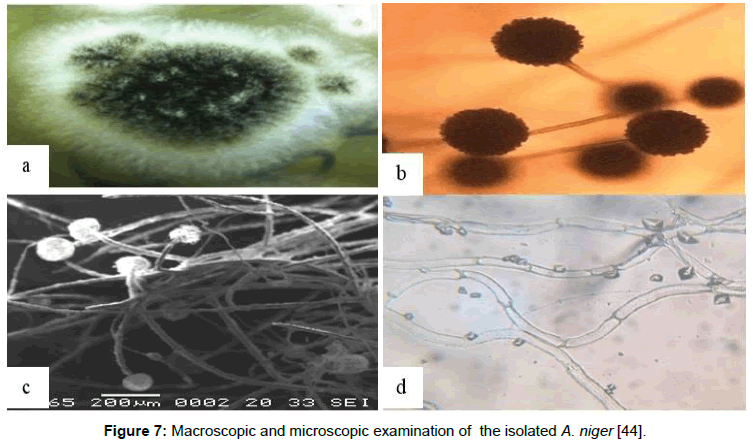
In the most recent publication, El-Sayyed et al . [44] studied the capability of Aspergillus niger for bioconcentrating radiocesium and/ or radiocobalt from the polluted waste stream and the tolerance of the fungi to operate in the radioactive environment for 190 days [7].
The data obtained from macroscopic (Figure 7a and 7b) and microscopic (Figure 7 c and 7d) examinations demonstrated that A. niger showed more tolerance for gamma irradiation and can work properly in the polluted solution for 190 days with total gamma activity from Co-60 and Ca-137 up to ~39 × 103 Bq and total irradiation dose ~4.5 × 1013 μS. This can be due to physiological adaptation of the microorganism and could be associated with increased tolerance for gamma irradiation and may be attributed to the melanin layer, (Figure 8), adhering to the conidial cell wall of A. niger.
Figure 7: Macroscopic and microscopic examination of the isolated A. niger [44].
Figure 8: Melanin formed in the spiked solution with Cs-137 and Co-60 compared to the control unspiked one post 190 days incubation period [44].
To save the environment, further studies as a subsequent stage following the remediation processes could be the solidification of the generated biological waste accumulated the heavy toxic metal or radionuclides using cementitious compounds as reported in various [49,50].
Conclusion
Although no one has found any permanent means for safe manipulation of radioactive pollutions, the nuclear industry continues to produce more and more, though, peaceful usages of radioisotopes in various fields of daily life, weapons research and production, and in nuclear power plants of all kinds. The industry mines, transports, processes, reprocesses, and buries nuclear materials with totally inadequate safeguards, threatening life and health at every step of the way. Secrecy and misinformation keep up public ignorance of the dangers. Citizens throughout the world must educate themselves and bring pressure to bear on governments and corporate interests to dismantle the improper danger applications altogether and provide for the safe, accessible storage and monitoring of all radioactive materials, so that future generations will be protected as much as possible and enabled to continue the guardianship of this legacy as long as necessary. Efforts to evaluate and refine current regulations must be steeped in the lessons learned and scientific evidence drawn from past accidents and incidents so that we can continue pursuing the safe and efficient use of nuclear technology.
The environmental impacts and costs of different methods for nuclear technologies must be considered. On the other hand, the rewards of nuclear technologies encounter additional challenges when omitting the dangers of radioactive by-products pollutions and nuclear terrorism. Also, the great worry about the highly radioactive wastes from nuclear power generation should be, through improved technical means, safely managed and stored such that keeping our land clean and tidy for us and the coming generations.
The present treatment technologies have to be updated, by means, to upgrade or modified, or redesign, or modernize by exploring materials and methodologies which are more effective, inexpensive, and unsophisticated.
However, the growing awareness and the tough laws concerning the environmental protection has encouraged the development of more eco-friendly technologies and providing useful information on candidate methods based upon the use of biological agents for remediation of radioactive pollutants including bacteria, yeast, fungi, algae, and plants. More studies are needed to evaluate the combination of more than one strategy for efficient removal of pollutants generated from anthropogenic activities with minimal environmental impact.
Public acceptance should put forward in the decision-making procedure for remediation and development technologies in the radioactive pollution management and build upon on that they or their environment will not be harmed and the radioisotopes applications will be safe.
References
- Posudin Y (2014) Methods of measuring environmental parameters. John Wiley and Sons.
- Pravalie R (2014) Nuclear weapons tests and environmental consequences: A global perspective. Ambio 43: 729-744.
- Rao KR (2001) Radioactive waste : The problem and its management. Curr Sci JSTOR 81: 1534-1546.
- Radioactive particles in the environment: Sources, particle characteristics, and analytical techniques (2011) IAEA-TECDOC Vienna 32.
- WHO (2013) Global Tuberculosis Report2013.
- Anna G, Nilb N, Suursoo S, Putk K, Kiisk M, et al. (2017) Regeneration of filter materials contaminated by naturally occurring radioactive compounds in drinking water treatment plant. J Water Process Engineer 30: 100464.
- Keepax RE, Moyes LN, Livens FR (2009) Speciation of heavy metals and radioisotopes. Environ Ecol Chem Sabljic A Ed. Eolss Publ. Co. Oxford, UK. 2, 165-199.
- Ješkovský M, Lištjak M, Sýkora I, Slávik O, Povinec PP (2018) Anthropogenic 137Cs on atmospheric aerosols in bratislava and around nuclear power plants in slovakia. J Environm Radioactivity 184: 77-82.
- A basic toxicity classification of radionuclides (1963) Technical Report Series 15, IAEA.
- Valentin J (2008) The 2007 Recommendations of the International Commission on Radiological Protection. Elsevier International Commission on Radiological Protection.
- Sidney K (2014) The Chemistry and toxicology of depleted uranium. Toxics 2: 50-78.
- Katherine Z, Jadvar H, Capala J, Fahey F (2016) Targeted radionuclide therapy: practical applications and future prospects: supplementary issue: biomarkers and their essential role in the development of personalised therapies (A). Biomarkers in Cancer 8.
- Maxwell O, Wagiran H, Zaidi E, Joel ES, Tenebe IT, et al. (2016) Radiotoxicity risks of radium-226 (226 ra) on groundwater-based drinking at dawaki, kuje, giri and sabon-lugbe area of abuja, north central Nigeria. Environ Earth Sci 75: 1084.
- Rakmetkazhy BI, Bulgakova O (2015) The health effects of radon and uranium on the population of Kazakhstan. Genes and Environment 37: 18.
- Geoffrey EG (1978) Hazards and control of ruthenium in the nuclear fuel cycle. Prog Nucl Ener 2: 29-76.
- Ivana S, Šljivić-Ivanović M (2016) Radioactive contamination of the soil: Assessments of pollutants mobility with implication to remediation strategies. Soil contamination-current consequences and further solutions. IntechOpen.
- Ulrika B, Lipponen M, Auvinen A, Jokiniemi J, Zilliacus R (2004) Ruthenium behaviour in severe nuclear accident conditions. Final Report. Nordisk Kernesikkerhedsforskning
- Silini G, Metalli P, Vulpis G (1973) Radiotoxicity of Tritium in mammals. Critical analysis of the extrapolation to man of the results of tritium incorporation into animal tissues. Eur 5033.
- Poliakova I (2017) Toxicity of radionuclides in determining harmful effects on humans and environment. J Environ Sci Public Health 1: 115-19.
- Hasegawa A, Tanigawa K, Ohtsuru A, Yabe H, Maeda M, et al. (2015) Health effects of radiation and other health problems in the aftermath of nuclear accidents, with an emphasis on fukushima. The Lancet 386 : 479-488.
- Revkin A (2012) Nuclear risk and fear, from hiroshima to fukushima. dot earth. opinion pages. The new york times. Retrieved march 31: 2012.
- Hippel V, Frank N (2011) The Radiological and psychological consequences of the fukushima daiichi accident. Bulletin of the Atomic Scientists 67: 27-36.
- Remediation of sites with dispersed radioactive contamination (2004) Technical report series 424, Internat Atomic Energy Agency.
- Jie L, Wang X, Zhao G, Chen C, Chai Z, et al. (2018) Metal-organic framework-Based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chem Soc Rev 47: 2322-2356.
- Dulama M, Deneanu N, Pavelescu M, Pasăre L (2009) Combined radioactive liquid waste treatment processes involving inorganic sorbents and micro/ultrafiltration. Rom J Phys 54: 851-859.
- Gangadharan A, Jasu N, Wadhwa S, Benny G, Tomar NS, et al. (2013) Management of intermediate level radioactive liquid waste (ILW) at WIP, Trombay, BARC Newsletter.
- Atomic I, Agency E. Handling and processing of radioactive waste from nuclear applications.
- Ghattas NK, Eskander SB (1997) New technology for the treatment of low and intermediate level radioactive organic waste from nuclear applications. U.S. Department of Energy Office of Scientific and Technical Information
- Eskander SB, Khalil LH, Sabry DY (2000) Ion-exchange characteristic of carboxymethylated cross-linked pregelled starch removal of Co-60 and Cs-137 from aqueous waste solution. Seventh Conference on Nuclear Science and Application, Cario, Egypt.
- Tawfik ME, Eskander SB, Elsabee MZ (2015) Extracted chitosan as biosorbent for" 6" 0co and" 1" 3" 7cs from radioactive waste simulates. Int Nucl Info Sys, IAEA
- Eskander SB, Bayoumi TA (2015) Biosorption of 137 Cs and/or 60 Co from Radioactive waste solution simulates using spent black tea (Camellia sinensis) Dregs. Int J Mat Chem Phy 1: 333-342.
- Viglašová E, Daňo M, Galamboš M, Rosskopfová O, Rajec R, Novák I (2016) Column studies for the separation of 99m tc using activated carbon. J Radioanalytical Nucl Chem 307: 591-597.
- Kumar A, Bisht BS, Joshi VD, Dhewa T (2011) Review on bioremediation of polluted environment: A management tool. Int J Environm Sci 1: 1079.
- Azubuike CC, Chikere CB, Okpokwasili GC (2016) Bioremediation techniques-Classification based on site of application: Principles, advantages, limitations and prospects. World J Microbiol Biotech 32: 180.
- Purohit J, Chattopadhyay A, Biswas MK, Singh NK (2018) Mycoremediation of agricultural soil: Bioprospection for sustainable development. Mycoremediation and Environmental Sustainability 91-120.
- Christopher RJ (2014) Mycoremediation (Bioremediation with Fungi)-growing mushrooms to clean the earth. Chemical Speciation and Bioavailability 26: 196-198
- Eskander SB, Abd El-aziz SM, Saleh HM (2012) Cementation of bioproducts generated from biodegradation of radioactive cellulosic-based waste simulates by mushroom. International Scholarly Research Network 2012: 6.
- Kulshreshtha S, Mathur N, Bhatnagar P (2014) Mushroom as a product and their role in mycoremediation. AMB Express 4: 29.
- Eskander SB (2001) Biological treatment of radioactive liquid waste simulate by pleurotus ostreatus. Isotope Rad Res 33: 193-200.
- Kamel HA, Eskander SB, Aly MAS (2007) Physiological response of epipremnum aureum for Cobalt-60 and Cesium-137 translocation and rhizofiltration. Int J Phytoremediation 9: 403-417.
- Eskander SB, Nour El-dien FA, Hoballa EM, Kh Hamdy (2011) Capability of lemna gibba to biosorb cesium-137 and cobalt-60 from simulated hazardous radioactive waste solutions. J Microbiol Biotech Food Sci 1: 148-163.
- Kamal KH (2012) Treatment and conditioning of hazardous and radioactive wastes. CU Theses.
- Aziz SMA, El-saayed H (2011) Bioaccumulation of Cesium-137 and Cobalt-60 from solid cellulosic-based radioactive waste simulates by plurotus pulmonarius. African J Microbiol Res 5: 2804-2811.
- El-Sayyad HE, Eskander SB, Bayuomi TA (2018) Capability of Aspergillus niger to Bioconcentrate Cesium-137 and Cobalt-60 from medium and low level radioactive waste solution simulates. Ind J Microbiol Res 5: 318-325.
- Saleh HM, Bayoumi TA, Mahmoud HH, Aglan RF (2017) Uptake of Cesium and Cobalt radionuclides from simulated radioactive wastewater by ludwigia stolonifera aquatic plant. Nucl Engineering Des 315: 194-199.
- Hosam SM, Aglan RF, Mahmoud HM (2019) Ludwigia stolonifera for remediation of toxic metals from simulated wastewater. Chem Ecol 35: 164-178.
- Saleh HM (2012) Water hyacinth for phytoremediation of radioactive waste simulate contaminated with cesium and cobalt radionuclides. Nucl Engineer Des 242: 425-432.
- Saleh HM (2014) Stability of cemented dried water hyacinth used for biosorption of radionuclides under various circumstances. J Nucl Mat 446: 124-133.
- Bayoumi TA, Saleh HM (2018) Characterization of Biological waste stabilized by cement during immersion in aqueous media to develop disposal strategies for phytomediated radioactive waste. Prog Nucl Ener 107: 83-89.
- Hosam SM, El-saied FA, Salaheldin TA, Hezo AA (2018) Macro-and nanomaterials for improvement of mechanical and physical properties of cement kiln dust-based composite materials. J Cleaner Prod 204: 532-541.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi