Research Article, J Nanomater Mol Nanotechnol Vol: 5 Issue: 3
Characterization and Cellular Fluorescence Microscopy of Superparamagnetic Nanoparticles Functionalized with Third Generation Nanomolecular Dendrimers: In-vitro Cytotoxicity and Uptake study
| Mohammad E. Khosroshahi1,2* and Maryam Tajabadi1 | |
| 1Laser and Nanobiophotonics Lab., Biomaterial Group, Faculty of Biomedical Engineering, Amirkabir University of Technology, Tehran, Iran | |
| 2Department of Mechanical and Industrial Engineering, University of Toronto, ON, Canada | |
| Corresponding author : Mohammad E. Khosroshahi Department of Mechanical and Industrial Engineering, University of Toronto, ON, Canada E-mail: khosrom@mie.utoronto.ca |
|
| Received: January 10, 2016 Accepted: May 12, 2016 Published: May 19, 2016 | |
| Citation: Khosroshahi ME, Tajabadi M (2016) Characterization and Cellular Fluorescence Microscopy of Superparamagnetic Nanoparticles Functionalized with Third Generation Nano-molecular Dendrimers: In-vitro Cytotoxicity and Uptake study. J Nanomater Mol Nanotechnol 5:3. doi:10.4172/2324-8777.1000186 |
Abstract
Characterization and Cellular Fluorescence Microscopy of Superparamagnetic Nanoparticles Functionalized with Third Generation Nanomolecular Dendrimers: In-vitro Cytotoxicity and Uptake study
An optimal sample was selected as those synthesized at 70ºC with particle size of about 10 nm << exchange length of 27 nm and a saturation magnetization of 67.8 emu/g. The samples were characterized with X-ray diffractometry (XRD), transmission electron microscopy (TEM), Fourier transform infrared (FTIR) spectroscopy, UV-vis spectroscopy, fluorescent spectroscopy (LIF) and magnetization measurements (VSM). The coated materials illustrated strong magnetic behaviour and XRD pattern like magnetite. The presence of Fe-O-Si bond in FTIR spectra confirmed the formation of thin APTS layer on the surface of magnetite nanoparticles. Thermogravimetric analysis (TGA) indicated that the modification of core synthesis technique can raise the efficiency of aminosilane coating reaction (as an initiator for PAMAM dendrimer) up to 98% with the production of about 610 dendritic arms. UV-vis spectrum of both SPIONs and ID-NPs was measured in the range of 340-380 nm with the maximum peak at about 350 nm. The fluorescence properties of ID-NPs distributed in a collagenous substrate and MCF 7 cells was studied by fluorescence microscopy. The results showed that the viability of L 929 and MCF 7 cells decreased from 100% and 90% to 53% and 23% respectively between 10 μg/mL and 1 mg/mL for ID-NPs. The rate of uptake increased with time and it was higher for ID-NPs than SPIONs.
Keywords: Magnetite core; PAMAM dendrimer; Fluorescence spectroscopy; Fluorescence microscopy; Cytotoxicity; Uptake
Keywords |
|
| Magnetite core; PAMAM dendrimer; Fluorescence spectroscopy; Fluorescence microscopy; Cytotoxicity; Uptake | |
Introduction |
|
| Magnetite, Fe3O4, is a common magnetic iron oxide that has a cubic inverse spinel structure with oxygen forming a fcc closed packing and Fe cations occupying interstitial tetrahedral sites and octahedral sites. They can have controllable sizes ranging from a few nanometers up to tens of nanometers which is smaller or comparable to a cell (10-100 μm), a virus (20-450 nm) a protein (5-50 nm) [1]. When the size of these nanomagnets becomes so small (<15 nm) they are considered as single magnetic domain where the magnetic moment of the particle as a whole is free to fluctuate in response to thermal energy. Under such a condition the nanoparticle is said to be superparamagnetic iron oxide nanoparticle (SPION), which lacks a hysteresis loop and posses high field irreversibility, high saturation field and extra anisotropy contributions [1,2]. | |
| Over the past decades SPIONs which possess extraordinary size and morphology dependent physical and chemical properties, have attracted world-wide research attention not only because of the unique properties but also for their biocompatibility and remarkable magnetic properties including chemical composition, granulometric uniformity, crystal structure, magnetic behaviour, surface structure, adsorption properties, solubility and low toxicity [3-6]. Factors important for biomedical applications of nanoparticles mainly include: biocompatibility, particles size with overall narrow uniform size distribution (particles less than 100 nm have longer circulation time, larger effective surface areas and low sedimentation rates), surface characteristics to provide easy encapsulation and protect them from degradation, stability and good magnetic response. | |
| Magnetite nanoparticles have potential biomedical applications where they can be used as contrast agents for bioimaging [7]. For example, recently Kim et al. [8] could engineer fluorescent dendritic nanobrobes to contain multiple organic dyes and reactive groups for target-specific biomolecule labeling and other groups such as Linna et al. [9] have suggested to employ dendrimers for visualization of their cellular entry and trafficking, cancer hyperthermia [10], and targeted drug delivery [11-13] and photodynamic therapy [14]. Various synthetic methods have been used to produce magnetite nanoparticles including coprecipitation [15], microemulsion [16], laser pyrolysis [17], and hydrothermal synthesis [18]. Other investigators have reported the parameters affecting the production of magnetic nanoparticles such as pH, alkaline species [19], reaction temperature [20]. Functional groups play an important role in the production of organic shell around inorganic core to prepare uniform and stable suspension [21,22] Dendrimers are a class of well-defined nanostructured macromolecules with a three-dimensional structure composed of three architectural components: a core (I), an interior of shells (generations) consisting of repeating branch-cell units (II), and terminal functional groups (the outer shell or periphery) (III) [23,24]. They are synthesized from a polyfunctional core by adding branched monomers that react with the functional groups of the core, in turn leaving end groups that can react again [25,26]. One such example is poly (amidoamine) (PAMAM), which acts as a template or stabilizer for preparation of inorganic nanocomposites. Some important properties of these structures include a large number of end groups, the functionable cores, the nanoporous nature of the interior at higher generations [24,27]. Also, their chemical structures, molecular weight, and molecular size can provide special type of functionality, which play a role in developing fields of materials science [28-31]. | |
| Intrinsic fluorescence of dendrimer minimizes the difficulties associated with the synthesis and purification of dendrimer based drug carriers attached with conventional type fluorescent molecules. As this matter reduces steps required for production of fluorescent carrier, the final biocompatibility of delivery system will be improved. More importantly, this will result in the enhanced drug loading capacity for cases where drugs are attached to the periphery of dendrimers, as available free space will be more due to the absence of conventional fluorophores in the system [32-34]. In this study, we report the results of i) Synthesis and characterization of SPIONPAMAM (ID-NPs), ii) Evaluation of cytotoxicity and uptake of SPIONs and ID-NPs by MCF 7 breast cancer and L929 cell lines based on the UV-vis absorbance spectroscopy and intrinsic fluorescent properties of these nanocomposites. | |
Materials and Methods |
|
| Synthesis of SPIONs | |
| Solutions of ferric chloride hexahydrate (FeCl3.6H2O, 99%, Merck) and ferrous sulfate heptahydrate (FeSO4.7H2O, 99%, Merck) were prepared as iron source in double distilled water. The optimized value of 0.9 M of ammonia solution (NH4OH-Sigma Aldrich) as alkaline source was used according to our previous report [35] and vigorously stirred under N2 bubbling at room temperature. The mixture of ferric and ferrous solutions was deoxygenated by bubbling N2 gas following sonication for 30 minutes. This solution was added drop wise to the stirring ammonia solution. The ferrofluid was prepared at two different temperatures. In the first group, the reaction temperature was kept constant at 25°C for 1 hour in a water bath before the mixture purified. The samples in the second group were mixed at the same condition (25°C for 1 hour) and then transferred to 70°C water bath under vigorous stirring for 30 minutes before purification. For both groups, the black precipitation was purified using magnetic separation five times and sedimented by centrifugation. The resultant material was dried by freeze dryer (Unicryo MC-4L) for 24 hours. | |
| Functionalization of SPIONs by Aminosilane (G0) | |
| A solution of optimal iron oxide sample with concentration of 2.13 mM was prepared in ethanol (149 mL), double distilled water (1 mL) and sonicated for 30 minutes. An amount of 35μL of aminopropyle triethoxysilane-H2N(CH2)3Si(OC2H5)3 (APTS, 99%, Sigma- Aldrich) was added to the mixture and stirred vigorously for 7 hours. It was then washed with ethanol five times using magnetic separation and finally sedimented by centrifugation. In order to remove the solvent, precipitated material was placed in freeze dryer for 24 hours. | |
| Synthesis of dendrimer functionalized SPIONs (ID-NPs) | |
| Formation of PAMAM dendrimer on the surface of amine-functionalized SPIONs was done according to the methods described by Liu and Pan [36,37] with some modification that we reported previously [38]. Dendritic polymer synthesis involves iteration of two main reactions which consist of two steps: Step one (Michael addition) alkylation of primary amines using MA (Methyl Acrylate, 99%, Aldrich), and Step two, amidation of the ester groups with Ethylene diamine (EDA, 99%, Sigma-Aldrich). Each Michael addition reaction produces a half generation of PAMAM dendrimer and amidation reaction creates the full generation. | |
| Characterization | |
| An X-ray diffractometer (Cu Kα, λ=1.5406 Å, FK60-40) was used to determine the crystalline phase of nanoparticles. Magnetic properties of the samples were measured by vibrating sample magnetometer (VSM-PAR 155) at 300 K under magnetic field up to 8 KOe. The presence of silane and PAMAM typical bonds on the surface of SPIONs was proved by Fourier transform infrared (FTIR) spectroscopy (BOMEM, Canada). Particle size and morphology of the nanoparticles were determined by transmission electron microscopy (TEM, Philips CM-200-FEG microscope, 120 KV). The amount of APTS and PAMAM molecules covered the surface of SPIONs was estimated using Energy-dispersive X-ray spectroscopy (SEM-EDS: Oxford Instrument– UK) and Thermogravimetric analysis (TGA50, Shimadzu, Japan). | |
| The absorbance spectra of magnetite and different generations of nanodendrimers were observed using SpectroFluorophotometer (RF-1510, Shimadzu, Japan). The evaluation of fluorescence emission of ID-NPs (G3) nanoparticles at different excitation wavelengths was performed using an ion argon laser Melles Griot-35MAP431). The fluorescence signals were detected by a 600 μm core diameter optical connected to spectrometer (UV-vis USB 4000, Ocean Optics). The excitation wavelengths covered a range between (454-514) nm. After obtaining the spectra of samples, they were smoothed by Gaussian model using Findgraph software. The next set up was to evaluate the efficiency of these nanoparticles in colouring the polymeric substrate, using two kinds of natural polymers, i.e., cotton and collagen. After the injection of nanodendrimers solution to these substrates, fluorescence microscopy (Zeiss Axioshop-Germany) was used to study the materials fluorescence). The Fe content of cells after uptake in each well was measured using JENWAY 6305 UV/Vis Spectrometer. | |
| In vitro cytotoxicity evaluation | |
| MCF 7 (human breast cancer) and L929 (the primary mouse connective tissue cells) cell lines were obtained from the National Cell Bank of Iran (NCBI) Pasteur Institute. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and were seeded onto a glass cover-slips in a 96 well-plate at a density of 15,000 cells per well in 100 μL of medium at 37°C in a 5% CO2 incubator for 24 h. Serial dilutions of ID-NPs (10 μg/mL-1000 μg/mL) were then added to each well. The control well represented the cells in culture medium without any nanoparticles. After 24 h of incubation of cells with ID-NP samples, all culture medium was replaced by 100 μl of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide, Sigma-USA). After 4 h the formation of formazan crystals was checked by optical microscope and the supernatant was removed. In order to dissolve formazan crystals, 100 μl of isopropanol was added to each plate and they were placed inside an incubator (Memmert-GmbH) for 15 minutes. The absorbance of each well was read using microplate reader (stat fax-2100, AWARENESS, Palm City, USA) at 545 nm. The results were reported in the form of relative cell viability compared to control sample (which could be calculated by the following equation): | |
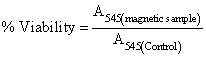 (1) (1) |
|
| Cell morphology and surface absorbed nanoparticles were detected by Scanning Electron Microscopy (SEM). | |
| Uptake rate of ID-NPs | |
| Nanoparticles play an important role in current cancer research, and the interaction of cells with nanoparticles is of particular interest. For this purpose, 105 L929 cells per mL of culture medium were seeded in 12 well-plate 24 hrs before addition of nanoparticles. 100 μL of ID-NPs with concentration of 100 μg/mL were added to each wells and samples were incubated for 4 and 10 hrs at 37°C in a 5% CO2 incubator. The control sample represented the culture medium without any nanoparticles. After that, cells were washed twice with 100 μL fresh PBS and the resultant solution was placed in another fresh 12-well plate. The cell membranes were dissolved by addition of acetic acid 5% and the resultant absorption was recorded as up taken nanoparticles by the cells. The supernatant UV-Vis absorption of each samples were read at 3 major characteristics peaks of Fe3O4 (344, 354 and 373 nm) to evaluate the extent of Fe content of cells in each well. The absorption was read using JENWAY 6305 UV/Vis Spectrometer. The Fe content (%) was measured by following equation: | |
| Here, I373 (Control) is the absorption regarding supernatant of control samples at 373 nm [39,40], Itest is the supernatant absorption of cells which contain magnetic nanoparticles after addition of acetic acid. | |
| Cellular scanning electron microscopy | |
| The L929 and MCF7 cells were cultured in 12 well-plates for 24 hours. Two different concentrations of 50 and 100 μg/mL of ID-NPs were added to each well and they were incubated for 8 and 24 hours. The cells were then fixed with 4% gluteraldehyde (Sigma, UK) buffered in PBS at room temperature for one hour. The cellular up take of nanoparticles at different concentrations and time were studied by SEM (XL30, Philips, USA). | |
| Cellular fluorescence microscopy | |
| Prior to this experiment, the fluorescent properties of the ID-NPs were studied using a concentration of 50 μg/mL in culture medium as a control sample. The same concentration was also suitably soaked in cotton and collagen substrates and the experiment was repeated. The fluorescence was observed using a fluorescence microscope (Axioscop, Zeiss, Germany). Next, the L929 and MCF7 cells with concentration of 104 per test were seeded in FBS pretreated slides containing collagen as a substrate for the cells deposition purpose. The ID-NPs were then added to the cultured cells and after 24 hrs, the medium was removed and the cells were rinsed with phosphate buffer saline (PBS). The fluorescent emission of the samples was detected as before. | |
Results and Discussion |
|
| SPIONs | |
| It is known that the particles size and shape strongly depend, among other factors, on the balance of nucleation and growth rates [35,41,42]. When ingredients of reaction are added together, the nucleation phenomena occur at supersaturation state. In our case, in order to produce tiny nuclei, the initial temperature of this study was set to 25°C and after 1 hour group 1 was purified which resulted in particles with larger size (average diameter is about 14 nm). Figures 1A & 1B show respectively the slight decrease of particle size and increase of saturation magnetization of SPIONs at elevated temperature because of loss of surface defects. Given that the completeness of diffusion and growth processes of samples are influenced by the reaction time [43,44], in our case, this was achieved at an elevated temperature of 70°C and prolonged time where the samples with less crystal defects and smaller particle size (average diameter is about 10 nm) were produced. With increase of ingredient (alkaline media) concentration more materials are available on the growth phase and thus particles with higher diameter could be obtained (Figure 1B). With increase of alkaline media concentration, the probability of non-magnetite layer production (magnetically dead layer) increases with decrease of saturation magnetization which indicates there is a limit for positive effect of alkaline media on saturation magnetization (Figure 1) | |
| Figure 1: Variation of magnetite particle size (a) and saturation magnetization (b) with alkaline and salt concentration for different synthesizing temperatures. | |
| In magnetic materials the magnetic properties such as saturation magnetization, susceptibility and coercivity tremendously are affected by particle size. Figure 2 illustrates the variation of coercivity of synthesized SPIONs with alkaline concentration at a given Fe concentration and temperature. It appears that at lower temperature, the coercivity shows a little change whereas at higher temperature the change is more significant. | |
| Figure 2: Dependence of coercivity of magnetite nanoparticles on alkaline media concentration. | |
| Therefore, the results suggest that coercivity is strongly size dependent and bulk samples with sizes greater than the domain wall width can cause magnetization reversal due to domain wall motion. As domain walls move through a sample, they can become pinned at grain boundaries and additional energy is needed for them to continue moving. Pinning is one of the main sources of the coercivity. Grain size dependence of coercivity and permeability (GSDCP) theory [45,46] predicts: | |
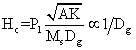 (3) (3) |
|
| Where Hc is the coercivity, A denotes the exchange constant, K is a magneto crystalline anisotropy constant, Ms the saturation magnetization, P1 and P2 are dimensionless factors. Therefore, reducing the grain size, Dg, creates more pining sites and increases Hc. For ultrafine particles, the modified form of theory predicts: | |
| Where P1 and P2 are dimensionless factors. The difference between equations (3) and (4) is defined by ferromagnetic exchange length as: | |
| Using the following parameters for Fe3O4 (K=1.35×104 J/m3, A=10-11J/m), the exchange length can be estimated as Lex=27 nm [47]. | |
| SPION-PAMAM | |
| XRD: Crystalline structure of SPIONs were analyzed by XRD shown in Figure 3. The results confirmed the formation of highly purified magnetite phase of iron oxide with inverse spinel structure (JCPDS file no.19-0629) without any interference with other phases of FexOy. The XRD graph of APTS-coated SPIONs shows no significant change in crystalline structure except a slight decrease in the Bragg peak intensity at 511 and 440 Miller indices planes. However, a further decrease of peaks were noticed when the SPION-APTS nanoparticles were coated with PAMAM likely due to amorphous nature of dendrimer covering the surface of underlying layers. The corresponding particle sizes of magnetite, APTS-coated and PAMAMgrafted magnetite nanoparticles were found 9.98 nm, 11.2 nm and 13.5 nm for, respectively using the Scherrer equation, | |
| Figure 3: XRD pattern of synthesized (a) SPION, (b) SPION-APTS and (c) SPION-APTS-PAMAM Nanodendrimers. | |
| Where θ is the Bragg angle, D is the mean diameter of particles, λ is the wavelength of incident X-ray and β is the full width at half height (FWHM) and the shape factor constant K is equal to 0.9. | |
| TEM: Morphology and mean particle size of prepared SPIONs synthesized at elevated temperature are shown in Figure 4A where the nanoparticles exhibited a quasi-sphere shape and have sizes ranging from 9.5 to 16 nm. As it is seen, magnetic nanoparticles are aggregated and form a cluster, which makes it hard to determine their geometrical shape. The aggregation, results mainly from high surface-tovolume ratio, increased dipole-dipole interactions, the freeze-drying and sample treatment procedures. Figure 4B indicates the particle size distribution from 5 to 16 nm with a normal distribution fit where the mean particle size is about 10 ± 2.1 nm. However, he polydispersivity is much lower than those nanoparticles prepared at lower temperature because of reduced surface defects at higher temperature [30]. There is a critical size which defines the superparamagnetic region approximately defined by Vp ≈ 25kT/K where k is Boltzmann constant, K denotes anisotropy constant ( Fe3O4 = 1.35×10 J/m3) and T is the absolute temperature [48]. At room temperature, 300 K, the critical size is equal to 27 nm hence confirming the results. Figures 4C & 4D represent the SPION coated APTS and SPION-PAMAM respectively with the corresponding size distribution illustrated in Figure 4E. | |
| Figure 4: TEM images showing (a) SPIONs of diameter 9.5 ± 1.4 nm and (b) its corresponding size distribution, (c) SPION-APTS and (d) SPION-APTS-PAMAM nanodendrimers and (e) the corresponding size distribution of (d). | |
| VSM: Figure 5 indicates the VSM results determined for all the magnetic nanoparticles. It confirms the supermagnetism behaviour of the materials and that the maximum Ms value of 68 emu/g for SPIONs gradually decreases to about 62 emu/g for PAMAM-grafted magnetite nanoparticles due to hindering effect of non-magnetic layers, cation distribution and spin effects on the surface of core [17,49]. To prove this assertion, FTIR spectroscopy was performed. | |
| Figure 5: VSM of (1) SPION, (2) SPION-APTS and (3) SPION-APTS-PAMAM Nanodendrimers. | |
| FTIR: Figure 6 shows the absorption bonds at 420 and 446 cm-1 and strong peaks around 581and 629 cm-1 , which confirm the formation of magnetite nanoparticles [50,51]. The peak at 993 cm-1 (G0 curve) is related to Si-O-Fe bonds and the stretching vibration of C-N bond, which overlaps with stretching vibration of Si-O observed at 1048 cm-1 [52,53]. The peak at 1151cm-1 (PAMAM-SPION) indicates the presence of C=O bond in ester groups [54]. In these spectra two intense peaks at 1570 cm-1 and 1631 cm-1 are observed that can be assigned to the N-H bending/C-N stretching (amide II) and C-O stretching (amide I) vibration of PAMAM dendrimer, respectively [55-58]. | |
| Figure 6: FTIR spectra of APTS and PAMAM-grafted magnetite nanoparticles at different stages. | |
| TGA: One trustworthy way of quantifying the number of coated molecules on the surface of nanoparticles is thermographic analysis (TGA). Figure 7 presents TGA curve of magnetite nanoparticles modified with APTS and PAMAM dendrimer. As can be seen in this Figure, the APTS-coated compound lost 8 percent of its weight at 700 °C. While the weight lost began at about 300◦C. This may be explained by vaporization of organic component. Since the mean diameter of the magnetite nanoparticles was known and there was no weight loss for unmodified magnetite nanoparticle, so the number of APTS molecules calculated according to the following formula [59]: | |
| Figure 7: TGA curve of magnetite nanoparticles modified with different generation of dendrimer. | |
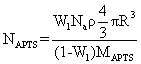 (7) (7) |
|
| Here NAPTS is the APTS number on each particle, Na=6.22 × 1023 is Avagadro’s number , Wl is the weight loss, ρ is the density of magnetite (5.17 g/cm-3), and MAPTS=221.37 is the relative molecular weight of APTS. As a result, it can be estimated that 610 APTS molecules i.e., amine groups are available on the surface of each coated Fe3O4 nanoparticle which effectively could participate in the reaction leading to the formation of dendritic arms for PAMAM dendrimer coating on the surface of SPION nanoparticles. | |
| The TGA results are similar to previous studies related to synthesis of PAMAM dendrimer [56,60]. The percent of weight loss was increased with increasing the dendrimer generation number which is expected due to the increase in chain length of C-backbone and molecular weight of dendrimers (compared to the lower generations). The remaining quantities may manifest the residual inorganic content due to the existence of iron oxide phase. Our findings are in good agreement with Niu, et al. report [61] who declared ester terminated products (half generation) are thermally stable under 200°C. Clearly, the TGA curves of PAMAM dendrimer have two stages of weight loss which was observed and reported before [54]. At the first stage, the formation of hydrogen bonds between the amine groups leads to the increase in viscosity of G1, G2 and G3 which made the complete removal of amine groups difficult at first stage. The second stage accounts for the decomposition of the dendrimer structure. TGA graphs show the average weight loss of about 8%, 11.5%, 24.3%, and 41% for different generations of dendrimer grafted magnetite nanoparticles (generation 0 to 3, respectively). | |
| UV-vis and fluorescence spectroscopy: Figure 8 shows the absorption peaks for the UV-vis spectrum of magnetite nanoparticles in aqueous ammonia solution in the range of 340-380 nm where the maximum occurs at about 350 nm. In general, the fluorescence behaviour in various solvents can reflect the interaction between the solvent and fluorophore. With formation of polymeric layer on the surface of Fe3O4 core, the intensity of the peaks correspondingly decreases at higher generation order. There are three types of electronic transitions: (1) FeIII crystal or ligand field transitions, (2) interactions between magnetically coupled FeII ions and (3) Oxygenmetal charge transfer excitations from the O(2P) non-bonding valence bands to the Fe(3d) [62]. The absorption band in the region of 330-450 nm originates from primarily from the absorption and scattering of UV radiation by magnetic nanoparticles. The absorption band at about 320-370 nm indicates the formation of nanosized particles. The intensity of the light scattered depends on the polarizability and that in turn depends on the molecular weight. This property of light is a valuable tool for measuring molecular weight. Considering the significance of the mean cosine scattering angle θ (anisotropy factor), |
|
| Figure 8: Absorbance spectroscopy of magnetite and PAMAM dendrimer nanoparticles (G1,G2 and G3). The inset indicates the EDS result of magnetite nanoparticles. | |
| For the magnetodendrimers experiment, ethanol was used as solvent. Alince PAMAM has fluorescent property due to -NH2, -OH and –COO-, therefore, it is an excellent way of recognizing the formation of the dendrimers moieties on the surface of the magnetic core. The concept of intrinsically fluorescing dendrimers which exhibit an unusual luminescence in the visible region, in the absence of conventional fluorophores, was initially reported for carboxylate terminated PAMAM dendrimers [27,63]. The results of laser-induced spectroscopy (LIF) are shown in Figure 9 where it is seen that the intensity amplitude of output emission signal increases with increasing the excitation wavelengths between (454-514) nm, Figure 9A. The corresponding fluorescence emission shown in Figure 9B covers a range between (490-550) nm with a significant bandwidth between 480-500 nm where 450 nm peak is assigned to carboxcilic acid and 470 nm to -NH2 bond. Clearly, there are three pronounced emitted peaks observed at 488, 495 and 550 nm. It is well known that the lower generation dendrimers are highly asymmetric and tend to exit in relatively open forms. As the generation number increases, their dendric conformations gradually approach towards a global shapes and covered by densely exterior groups. These changes in molecular conformation of higher generations favor a π-π interaction between phenyl rings, which leads to the formation of phenyl excimer. Thus, G3 displays an enhanced structureess emission due to the excimer formation. Most of the densely packed phenyl form parallel arrangement due to the reduced distance between phenyl rings leading to a stronger π-π interaction. It is interesting to note that with the generation increasing, functional groups attached to dendrimer surfaces can interact with one another and exhibit new functions [64]. The fluorescence intensities differ across all emission wavelengths for each of the spectra shown and it is evident that the number of photons in increase as the fluorescence wavelength increases (hence, decrease of photon energy). A close approximation of quantum yield can be obtained using, | |
| Figure 9: Laser-induced fluorescence spectroscopy of third generation PAMAM (G3) (a) Variation of intensity of emitted wavelengths with excitation wavelengths and (b) the emitted wavelengths with excitation wavelengths. | |
| Q.Y=No. of photons emitted / No of photons absorbed (8) | |
| The photon energy for every wavelength is determined using, Ep=hν=hc/λ where h is planck’s constant and c is velocity of light and λ is laser wavelength. The number of photons for each corresponding wavelength can be determined from (Np=Laser power / photon energy). Using the data from Figure 10, a close approximation was obtained as 0.93. | |
| Figure 10: Fluorescence microscopy of (A) Collagen substrate embedded by ID-NPs, (B) MCF 7 cells seeded in collagen substrate containing ID-NPs. The samples (a,e), (b,f), (c,g) and (d,h) are control and excited samples at different wavelengths using Blue, Green and Red filters respectively. | |
| To explain such a wide spectrum, it is noteworthy that there are three kinds of functional groups existing in NH2-terminated PAMAM dendrimers: amides, internal tertiary amine groups, and terminal primary amines and normally they exhibit photoluminescence spectra in a broad range increasing. The excitation wavelength results in red-shift of emission peaks, which can cover the entire visible region due to high structural heterogeneity and the broad molecular weight distribution of the hyperbranched PAMAMs [33] and also, electron-hole recombination processes involving correlated electronhole exciton states between localized states of electrons and holes [65]. | |
| Prior to evaluation of cytotoxicity and cellular uptake of the nanocomposites, their fluorescence properties were tested using cotton and collagen fibers as substrates as explained above. Figure 10A illustrates the fluorescence of ID-NPs distributed in collagen substrate and Figure 10B shows the same experiment results with MCF 7 cells using blue, green and red filters of fluorescence microscope. Considering the chemical structures of collagen and cotton, there is a possibility of hydrogen interaction between the hydroxyl group of the substrate and amine group of dendrimer nanocomposites which produces a new fluorescent moieties with a time dependent behaviour. The longer the time, the more fluorescent moieties is produced hence; more intense fluorescence image could be obtained. | |
| Cytotoxicity and uptake evaluation | |
| The cytototoxicity of SPIONs and ID-NPs was determined by colorimetric MTT assay experiments. The interaction of mitochondrial dehydrogenase in living cells with MTT oxidizes tetrazolium salt, which results in formation of a dark blue formazan product. The dehydrogenase activity of damaged or dead cells is noticeably lower than the normal cells [66,67]. Figure 11 represents the histogram plot of two cell lines viability (L 929 and MCF7) cultured with different concentration of SPIONs (Figure 7A) and ID-NPs (Figure 7B). The cell viability was studied after 24 hours incubation at 37°C and the calculations are based on the absorbance of samples at 545 nm and normalized using the equation (1). In the first case (i.e., SPIONs), the viability of MCF 7 and L 929 cells remains above 100% at 10 μg/mL respectively but for L 929, it decreases to about 78% at 1mg/mL. As in the second case (i.e., ID-NPs), the viability decreased from (90 to 23)% and from (100 to 53)% at corresponding values of 10 μg/mL and 1 mg/mL for MCF 7 and L 929 cells respectively. This clearly, confirms the concentration and cell type dependence of cytotoxicity. These findings are in good agreement with those reported by Jevprasesphant et al. [68] and Sgouras, et al. [69] regarding the cytotoxicity of third generation of dendrimer in the absence of magnetite nanoparticles. | |
| Figure 11: MTT analysis of (A) Magnetite and (B) SPION-PAMAM dendrimer (G3) nanoparticles in L 929 and MCF 7 cells at different concentrations. | |
| The decrease in cell viability with increase of nanoparticle concentration in the medium is more likely due to the enhanced uptake and the surface coverage of cells as a result of interaction between the cationic periphery of PAMAM-grafted magnetite nanoparticles and negatively charged cell surfaces [70]. The lower compatibility of MCF 7 compared to L 929 is partly because of nanoparticles attachment on the surface as seen by SEM in Figure 12 and higher rate of internalization of nanoparticles by the endocytosis pathway, which is clearly observable form the fluorescent images shown in Figure 13. This is further discussed in the following section. | |
| Figure 12: SEM micrograph of cellular distribution of SPION-PAMAM nanodendrimers in (a) MCF 7 and (b) L 929 cells using a concentration of 50 μg/mL. | |
| Figure 13: Fluorescence microscopy of cellular distribution of SPION-PAMAM nanodendrimers (50 μg/mL) in L 929 and (B) MCF 7 cells using Blue, Green and Red filters respectively. | |
| The last stage of the experiment was to evaluate the uptake of SPIONs and ID-NPs by MCF 7 cells by UV-vis absorbance spectroscopy at three main iron oxide absorption peaks as explained earlier. The Figure 14 clearly illustrates that uptake increases with time and that it is in order of ID-NPs>SPIONs for 344 nm>354nm>373 nm respectively. This finding can have an important impact on the cancer therapy in nanomedicine. | |
| Figure 14: Uptake level of Fe3O4 and G3 nanoparticles by MCF 7 cell lines. | |
| To explain and justify the mechanism behind the higher rate of ID-NPs uptake compared to SPIONs one can use the concept of the Zeta potential ξ, quantitatively without going through the vigorous mathematical treatment. Basically, ξ is an electrostatic potential that exists at the shear plane of a particle and is related to both surface charge and the local environment of the particle. Zeta potential has been used in cell biology to study cell adhesion, activation, and agglutination based on cell-surface-charge properties [71-75]. Nanoparticle uptake by cells is considered a two-step process: i) binding of nanoparticles to the cell surface, followed by ii) the internalization of nanoparticles by the specific endocytosis pathway. The relative Zeta potential Zr of cells during the adsorption of nanoparticles is expressed as: | |
| where mbinding is the total mass of nanoparticles binding to the cell surface, mint is the total mass of nanoparticles internalized within the cell and β measures how the presence of nanoparticle adsorption affect the free energy of the ions at a distance from the cell surface. Thus, when nanoparticles bind onto the negatively charged cell surface with the same sign of ξ, it increases the free energy of the ions at a distance from the cell surface, so the β binding is positive i.e., Zr > 1. However, in our case, the PAMAM functionalized SPIONs have measured positively charged ξ, which is opposite to ξ sign of the cells. Therefore, the free energy of the ions at a distance from the cell surface is decreased and β binding is negative i.e., Zr<1 and the internalization mechanism dominates. Since, during the internalization of nanoparticles by the endocytosis pathway βint is positive i.e., Zr >1, the position of the plane of shear is shifted and the negative surface charge is increased compared to the binding process. The binding and internalization processes could be modeled by Langmuir adsorption [75,76]. In short, when the binding effect dominates, the cell’s negative ξ will first increase with time and then decrease but when the internalization effect dominates during the whole adsorption process, the cell’s negative ξ will decrease to a stable stage. | |
Conclusion |
|
| SPION-based third generation PAMAM nano-molecular dendrimers were synthesized and characterized. It was shown that these nanocomposites can possess a large number of dendritic arms which, can be used for conjugation of variety of moieties for different applications. The cytotoxicity and uptake of these nandendrimers with low coercive, high saturation magnetism core and wide range fluorescence spectrum were investigated using SEM and fluorescence microscopy. The results showed that the viability of MCF 7 breast cancer and L929 fibroblast cell lines decreased from (90 to 23)% and from (100 to 53)% at corresponding values of 10 μg/mL and 1 mg/mL respectively. The lower compatibility of MCF 7 compared to L 929 is thought to be due to internalization mechanism of these nano-molecular composites via the endocytotic pathway. Therefore, we suggest that these nanodendrimers can be used for multi-modal nanomedicine applications such as targeted therapy, drug delivery and bioimaging. | |
Acknowledgments |
|
| Authors greatly appreciate Dr Sh. Bonakdar from Pasteur Institute, National Cell Bank of Tehran for his help and consultance in preparation of cells. | |
References |
|
|
|
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 



