Research Article, Vector Biol J Vol: 1 Issue: 2
Chikungunya and Dengue Risk Assessment in Greece
| Romeo Bellini1, Paolo Bonilauri2, Arianna Puggioli1*, Davide Lelli2, Paolo Gaibani3, Maria Paola Landini3, Marco Carrieri1, Antonios Michaelakis4, Dimitrios Papachristos4, Athanassios Giatropoulos5, Evangelos Badieritakis4, Bettina Maccagnani1, Mattia Calzolari2and Michele Dottori2 | |
| 1Department of Medical and Veterinary Entomology, Centro Agricoltura Ambiente G Nicoli, Crevalcore, Italy | |
| 2Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia Romagna “B. Ubertini” (IZSLER), Brescia, Italy | |
| 3Regional Reference Centre for Microbiological Emergencies (CRREM), Microbiology Unit, Azienda Ospedaliero-Universitaria di Bologna, Policlinico S Orsola–Malpighi, Bologna, Italy | |
| 4Department of Pesticides Control & Phytopharmacy, Laboratory of Biological Control of Pesticides, Benaki Phytopathological Institute, Greece | |
| 5Department of Entomology and Agricultural Zoology, Benaki Phytopathological Institute, Kifissia, Greece | |
| Corresponding author : Arianna Puggioli Department of Medical and Veterinary Entomology, Centro Agricoltura Ambiente “G. Nicoli, Via Argini Nord 3351, 40014 - Crevalcore, Italy Tel: + 30 051 6802240 E-mail: apuggioli@caa.it |
|
| Received: April 01, 2016 Accepted: April 20, 2016 Published: April 27, 2016 | |
| Citation: Bellini R, Bonilauri P, Puggioli A, Lelli D, Gaibani P, et al. (2016) Chikungunya and Dengue Risk Assessment in Greece. Vector Biol J 1:2. doi:10.4172/2473-4810.1000108 |
Abstract
Objective: The aims of the study were:
(1) To assess the mutated CHIKV E1-A226V and DENV II infection and dissemination rates of an Ae. albopictus population established in Athens (Greece)
(2) To assess the risk of outbreaks in four Greek localities based on Ae. albopictus population density whose estimate was based on the number of eggs laid in ovitraps.
Methods: Under laboratory conditions females were offered blood meal infected with the CHIKV titer of 1X106 TCID 50/mL and DENV II titer of 1.76 X106 TCID 50/mL; at day 11 after oral infection, females were sacrificed, legs were removed and processed for PCR analysis to assess the presence of viral replicates. In order to evaluate the risk of outbreak of CHIKV and DENV II, a pilot monitoring program was started in three Greek localities and in Chania (Crete), to estimate the adult female population density on the base of the number of eggs in the ovitraps.
Results: We proved the vector competence of the Greek Ae. albopictus strain for E1-A226V mutated CHIKV and DENV II. Combining the data on the vector competence with those on the female population density, based on the egg density data, the estimated risk of outbreak was relatively low but not negligible.
Conclusion: As the vector competence estimated under laboratory conditions was obtained by offering females moderately low initial virus titers, it can be expected a higher vector competence in the field. This consideration, together with a possible increase of the mosquito population due to the global warming effects, make the quantitative ovitrap-based monitoring a necessary and useful tool to estimate the risk of outbreaks.
Keywords: Aedes albopictus; Chikungunya; Dengue; Infection rate; Dissemination rate; Field risk assessment
Keywords |
|
| Aedes albopictus; Chikungunya; Dengue; Infection rate; Dissemination rate; Field risk assessment | |
Introduction |
|
| Globalization of trade and travel has facilitated the spread of nonnative species across the earth. A proportion of these species become established and cause serious environmental, economic and human health impacts. These species are referred to as invasive, and several invasive mosquito species (IMS) were inadvertently introduced in Europe, where they found favorable environmental conditions enhanced by the climate change [1]. The predicted increases in temperature (1.4 to 5.8°C by 2100) and rainfall are likely to extend the distribution of mosquitoes and associated pathogens, in addition to shortening the development time of mosquito larvae and the extrinsic incubation period of pathogens [1,2]. Warmer and wetter weather is likely to result in longer seasonal activity of mosquitoes, while sea level rise will produce new wetland habitats suitable as breeding sites by gradually inundating coastal regions [1,3]. The related sanitary risks include the reappearance of mosquito-borne diseases such as chikungunya, dengue, and West Nile fevers which are currently emerging in different European countries, and the possible emergence of new diseases, like those caused by USUTU virus, circulating among Ae. albopictus populations in Northern Italy [4] and by the possible diffusion of the zika virus (ZIKV) [5], for which Ae.albopictus vector competence is under investigation (author’s unpublished data). | |
| Aedes albopictus was first detected in Albania in 1979 [6] and its establishment has been reported in 13 further European countries to date: Croatia, France, Greece, Italy, Malta, Monaco, Montenegro, San Marino, Serbia, Slovenia, Spain, Switzerland and the Vatican City [7-9]. The species has also been detected in Belgium, Bosnia and Herzegovina, Germany and the Netherlands [8,9]. Distribution models predict that Ae. albopictus will continue to expand depending on transport, environmental and climatic changes [7,10,11]. After its first record in Northwest Greece in 2003-2004, Ae. albopictus occurs in many other Greek regions [12], with high populations detected in Athen’s urban areas [13]. This mosquito shows an aggressive nuisance behavior during the day and is considered competent to transmit at least 22 arboviruses [14]. The first chikungunya outbreak in a temperate region was reported from Northeastern Italy, with Ae. albopictus incriminated as the only vector [15]. Indigenous cases of chikungunya and dengue have been reported in France and Croatia in 2010 [16-18]. Up today, no chickungunya human case occurred in Greece, while in September 2012 the Hellenic Centre of Disease Control notified a case of dengue in an 84-yearold patient, who died. The most serious documented dengue virus epidemic in Europe occurred during the summers of 1927 and 1928, when about 90% of the population of Athens was infected and more than 1,000 persons died [19]. Aedes aegypti was responsible for those outbreaks, but in the last decades of the past century its presence had not been recorded in Europe. Now it is considered established in Madeira [20], Southern Russia, Abkhazia and Georgia [21]. Current models estimate some risk for dengue and chikungunya transmission in the Mediterranean basin related to climate change impact [22], as Ae. albopictus, whose vector competence has been demonstrated, could replace Ae. aegypti, being already present with abundant and increasing populations. | |
| Primary aim of this study is the assessment of the infection and dissemination rates [23,24] of the Ae. albopictus Greek strain for DEN II virus and for the CHIK E1-A226V mutated virus and the risk of outbreak depending on the vectorial capacity and on the density of Ae. albopictus population in Athens (Greece) and in Chania (Crete). | |
Materials and Methods |
|
| Infection and dissemination rates | |
| Trials were performed in a BSL-3 laboratory (28 ± 1°C, 80% RH, 14:10 L:D) by using Ae. albopictus females obtained from field collected eggs in Athens, Greece. The CHIKV isolate (GenBank, access code EU244823.2) was provided by IZSLER of Brescia (5° P Vero cell, initial titer 1X106 TCID50/mL), while the DENV II isolate was provided by CRREM of Bologna (7° P, initial titer 1.76 X 106 TCID50/mL). Three suspensions were prepared for each virus, as described in Table 1. At day 11 after oral infection, females were individually anesthetized on ice; legs were separated from body and separately stored into criovials containing 180 ul of PBS in liquid nitrogen. Details concerning rearing conditions, methods of females’ oral infection and quantitative virus analysis (QRT-real time PCR) are described in Bellini et al. [25]. The infection rate (proportion of females with virus positive bodies) and the dissemination rate (proportion of females with virus positive legs) were calculated. Paired comparisons (Fisher tests) were used to compare the number of positive/negative body samples (infection rate) and leg samples (dissemination rate) among the three virus titers of CHIKV and DENV II. | |
| Table 1: CHIKV and DENV II titers, preparation details and number of tested females (C= mutated CHIKV, and D=DENV II). The virus suspension was obtained by diluting the initial virus titer with the D-MEM substrate. | |
| Field risk assessment | |
| In order to estimate the risk of an outbreak to occur it is necessary to know the average number of bites per day per person and the vector competence for the specific pathogen. Statistically significant correlations were found between the mean number of eggs/ovitrap (which is the cheapest monitoring method for Ae. albopictus) [26] and the average number of bites per day per person estimated by Human Landing Collection (HLC). Therefore, the data obtained by an ovitrap-based monitoring system allow to estimate the number of biting females, a key factor for the calculation of the number of new cases which can arise from a singular case (R0), as follows [26]. | |
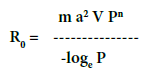 |
|
| m is the average number of bites per day per person based on the number of eggs per ovitrap; a2 indicates the propensity of the vector to bite humans; V is the vector competence; P is the daily survival rate of the vector; n is the length of the extrinsic cycle (the time from female infection to pathogen transmission). Epidemics occur when the calculated R0 value is higher than 1. In order to evaluate the risk of outbreak of CHIKV and DENV II, a pilot monitoring program was started in three Greek localities and in Chania (Crete), to estimate the adult female population density on the base of the number of eggs in the ovitraps. | |
Results |
|
| Infection and dissemination rates | |
| Figure 1 presents the rates of infection and dissemination obtained for the three CHIKV and DENV II titers. The infection rate for CHIKV decreased dramatically between C1 and either C2 or C3 (C1 vs C2, P = 0.0001; C1 vs C3, P = 0.0017), while no statistically significant difference was found between C2 and C3 (P = 0.4369). The decrease of the dissemination rate was less noticeable (C1 vs C2, P = 0.0174; C1 vs C3, P = 0.0640; C2 vs C3, P = 0.4470). The infection rates for DENV II were much lower than those measured for CHIKV and decreased significantly between D1 and D3 (P < 0.0001), D2 and D3 (Fisher test P < 0.0001), but not between D1 and D2 (P = 0.1444). The dissemination rate was not different between D1 and D2 (P = 0.6487), while it slowed down to zero for D3 (D1 vs D3, P = 0.1476; D2 vs D3, P = 0.1729). | |
| Figure 1: CHIKV and DENV II infection and dissemination rates in virus-exposed Ae. albopictus females (bars represents 95% confidence limits). | |
| Field risk assessment | |
| Based on egg density data, no risk of outbreak for the mutated CHIKV was found both in the Piraeus harbor (Port) and in the Athens International Airport “Eleftherios Venizelos” (Airport), while in Rizoupoli area (Athens) a low risk was found in August, 2014 and in the period July-October, 2015. In Chania (Crete) a low risk level was estimated in August-September, 2015 (Figure 2). In 2014 and 2015, no risk of outbreak was found for the DEN II virus in the four localities (Figure 3). | |
| Figure 2: Risk of outbreak for the mutated CHIK virus in four Greek localities (bars represent 95% confidence limits). | |
| Figure 3: Risk of outbreak for the DEN II virus in four Greek localities (bars represent 95% confidence limits). | |
Conclusion |
|
| The laboratory trials confirmed the vector competence of Greek Ae. albopictus females for E1-A226V mutated CHIKV and DENV II. At 11 days post-infection, the CHIKV infection rate was consistent with the results obtained with an Ae. albopictus strain from the Alpes-Maritimes (Southern France) [23]. The mutated CHIKV dissemination rate was very similar to the value estimated for the strain collected in Romagna (Northern Italy) during the 2007 CHIKV outbreak [24] and for the strain collected in La Reunion [26]. The results obtained for DEN II were consistent with results obtained by Vega-Rua et al. [23] at 9 days post-infection. In our study, the infection and the dissemination rates were lower than those of CHIKV confirming that Ae. albopictus is more efficient vector for CHIKV (mutated strain) than for DEN II. The risk of outbreak calculated in the Greek localities, based on the vector competence estimated for the Greek Ae. albopictus strain was relatively low, but we were able to test relatively low virus titers, which are likely to be higher under field conditions. This consideration, together with the likely increase of the mosquito population due to the global warming effects, and to the arboviruses’mutation capacity [27], make the quantitative ovitrap-based monitoring a necessary and useful tool to estimate the risk of outbreaks. Differences among virus titers, suspension preparation protocol, body parts used to estimate the dissemination rate, the day post-infection chosen for the tests may influence vector competence study results. | |
Competing Interest |
|
| The authors declare no competing interests. | |
Acknowledgments |
|
| This study has been conducted in the frame of the LIFE CONOPS project “Development & demonstration of management plans against - the climate change enhanced - invasive mosquitoes in S. Europe” (LIFE12 ENV/GR/000466) cofunded by the EU Environmental Funding Programme LIFE+ Environment Policy and Governance. We would like to acknowledge Dubravka Pudar for her precious help in the laboratory trials, and the generous assistance from Tania Zachariadou (Athens International Airport), Vassilio Kopelas (Piraeus Container Terminal) and the Directorate of Public Health (Region of Crete) in monitoring program. | |
References |
|
|
|
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 