Review Article, J Nanomater Mol Nanotechnol Vol: 12 Issue: 3
Fabrication and Application of Photocatalysis of Graphene and Graphene Derivative
Anjali Yadav1, Anamika Srivastava1, Bharti1, Sunidhia and Manish Srivastava2*
1Department of Chemistry, Banasthali University, Rajasthan, India
2Department of Chemistry, University of Allahabad, Prayagraj, India
*Corresponding Author: Manish Srivastava
Department of Chemistry, University of Allahabad, Prayagraj, India,
Tel: 917737559547;
E-mail: sagermanish1@gmail.com
Received date: 03 February, 2023, Manuscript No. JNMN-23-88613;
Editor assigned date: 06 February, 2023, PreQC No. JNMN-23-88613 (PQ);
Reviewed date: 20 February, 2023, QC No. JNMN-23-88613;
Revised date: 21 April, 2023, Manuscript No. JNMN-23-88613 (R);
Published date: 28 April, 2023, DOI: 10.4172/2324-8777.1000362
Citation:Yadav A, Srivastava A, Bharti, Sunidhia, Srivastava M (2023) Fabrication and Application of Photocatalysis of Graphene and Graphene J Derivative. Nanomater Mol Nanotechnol 12:3.
Abstract
Graphene is the most potential material in nanotechnology and has attracted a remarkable amount of attention in recent years in material science, due to its large specific area, unique monolayer structure, superior electron mobility, high conductivity, chemical stability, and electrical properties of the Two-Dimensional (2-D) material. The use of graphene and its derivatives to increase the effectiveness of photocatalysts has attracted much curiosity. This review focuses on the recent important development in the preparation and applications of graphene-based photo catalysts. The applications of the new materials in the degradation of pollutants, photocatalytic hydrogen evolution, and water disinfection are presented. High efficiency photocatalysts are vital for the removal of organic pollutants and environmental sustainability.
Keywords: Photo catalyst; Graphene-based photo catalysts; TiO2/graphene photocatalysts; Metal late/graphene photocatalysts; Application
Abbreviations:
GO: Graphene Oxide; GR: Graphene; RGO: Reduced Graphene Oxide; NPs: Nanoparticles; Rh B: Rhodamine B; LDH: Layered Double Hydroxide; TBOT: Tetrabutyl Orthotitanate; CVD: Chemical Vapor Deposition; P25: TiO2; BWO: Bismuth Tungstate; CNTs: Carbon Nanotubes
Introduction
Graphene, which is a single layered sheet of sp2 hybridized carbon atoms in a tightly packed 2-D honeycomb lattice. It has engrossed a great deal of research significance since its discovery in 2004 by Novoselov, et al. [1]. High Young’s modulus (≈1 TPa), excellent mobility of charge carriers (200,000 cm2V-1s-1), superior optical transparency (≈97.7%), superior mechanical stability, and large surface area (calculated value ~2630 m2/g) are the unique properties which are achieved by graphene [2]. The above mentioned properties result in the utilization of graphene in various fields such as catalysis, nano electronics and optoelectronics, drug delivery, supercapacitors, energy storage, and biosensors [3].
Graphene based composites are also highly desirable in the field of photocatalysis [4,5]. Photocatalysis has attracted intense attention during the past decades because of its potential for environmental purification and converting photon energy into chemical energy [6-9]. As the innovation of the photocatalytic splitting of H2O on electrodes of TiO2 in 1972 by Fujishima and Honda, significant progress has been done in highly active oxide semiconductor photocatalysts because of their ecological application [10-14]. This technology is based on the distinctive electronic structure of semiconductors, which is composed of a filled Valence Band (VB) and an empty Conduction Band (CB). Holes are produced in the valence band due to the excitation of electrons from the valence band to the conduction band on absorbing a photon of energy equal to or greater than the bandgap Energy (Eg) of the semiconductor [15]. Chemical oxidation or reduction process was triggered on the separation of photo-generated electrons and holes [16].
Photocatalytic conversion of carbon dioxide into hydrocarbon fuels, pollutants degradation, and water splitting for H2 generation are the various photocatalysis application. The area of graphene improved photocatalysis is moving ahead. In this review, we will focus on current advances in the preparation, design, and photocatalytic applications of graphene based composites [17].
Literature Review
Fabrication of graphene based photo catalysts
Graphene has been utilized in various semiconductors to prepare photocatalysts based on graphene. Metal oxides (TiO2, WO3, Cu2O, ZnO, Fe2O3, SnO2, NiO, ZrO2, MnO2, etc.), silver/silver halides (Ag/ AgCl and Ag/AgBr), sulfides (ZnS, SnS2, CuS, CdS, etc.), metal-free photocatalysts (g-C3N4 and α-S8) and oxy-acid salts (BiVO4, Bi2WO6, Sr2Ta2O7, Bi2 MoO6, ZnFe2O4, InNbO4, and LaMnO3, etc.) are mainly included by these photocatalysts. The ex situ hybridization approach and the in situ growth approach are the two main categories in which the synthetic methods are classified.
Ex situ hybridization strategy
The ex situ hybridization strategy involves the mixing of commercially accessible nanocrystals and graphene nanosheets in solutions. The graphene nanosheets or nanocrystals are frequently premodified to be enriched with functional groups to achieve interaction. For example, capped CdS quantum dots capped on Benzyl Mercaptan (BM) were resynthesized and then efficiently attached on RGO. This method involves the interaction of benzene rings with RGO via π–π stacking. Likewise, adhesive polymers are used for the premodification of GO/RGO sheets for anchoring the NPs. RGO surface was modified by Liu, et al., via the π–π interaction by employing amphiphilic biopolymers, like bovine serum albumin protein. Several types of metal NPs such as silver, gold, palladium, platinum are absorbed by the functionalized RGO which can be utilized as a universal adhesive layer.
In situ growth strategy
Weak interaction, non-uniformity, and particle aggregation resulted from an ex situ hybridization strategy between graphene oxide/ reduced graphene oxide. Charge transportation was limited between graphene and photocatalysts and results in poor activity. Photocatalysts based on graphene are prepared by the in situ growth technique.
Solution mixing and in situ growth: Satisfying results were obtained by solution mixing for the synthesis of graphene based photo catalysts. WO3/GR composite was prepared by Ng, et al., on mixing graphene oxide with WO3 powder to enhance the photocatalytic properties. WO3/GO dispersion was obtained by ultra-sonicated the mixture of graphene oxide suspension and WO3 for half an hour. WO3/ RGO composite was obtained by exposing the dispersion to either visible light or UV irradiation for 3 hours. Though the morphological feature of composites of WO3/RGO was the same, the particle size of WO3 was greater than 100 nm and tends to aggregate. The composites of WO3/RGO were synthesized by utilizing polyvinylpyrrolidone to combine reduced graphene oxide with tungsten by employing the similar procedure as above. The precursor solution of WO3/GO was obtained by ultra-sonicated and stirring the solution of GO and ammonium met tungstate hydrate. The precursor was calcinated for 5 h at 450℃ in the air to obtain the composite of WO3/RGO. Hence, the nano crystallites of WO3 ranged in 20 nm-40 nm were distributed consistently on the RGO.
NiTi-LDH/RGO catalysts derived by visible light were prepared by in situ technique by anchoring NiTi-LDH nano sheets on the surface of RGO. The monolayer suspension of RGO was formed by sonication in the deionized water. The sources of nickel and titanium and urea were mixed in the suspension of RGO. The suspension was stirred at 90℃ and the product obtained was vacuum dried in an oven for a day at 60℃. The nano sheets of NiTi-LDH were highly dispersed on the surface of RGO with a lateral diameter of 100 nm-200 nm and have a plate-like morphology. The homogeneous distribution of both titanium and nickel was displayed by the elemental mapping images.
Hydrothermal and solvothermal approach: The hydrothermal approach is an effective technique for the preparation of graphene based photo catalysts. The solvothermal and hydrothermal methods are more efficient in comparison to the solution mixing method due to their controllable morphology of semiconductor particles. NPs would be well bonded to graphene still without any intermediate. Α-Fe2O3/RGO composite was fabricated by Meng, et al., by using a hydrothermal approach in which hematite NPs are supported on nanosheets of RGO. The mixture was prepared by dissolving an appropriate amount of FeCl3.6H2O and graphene oxide in the distilled water and then it was sonicated. Ethanol was mixed in the prepared solution and thermal hydrolysis occurs on placing it in a boiling aqueous bath. The product obtained from the centrifuging was heated at 350℃ in the air for 120 minutes and then in pure N2 for 15 minutes at 800℃. It was observed that the crystalline size of monolithic α-Fe2O3 was greater than α-Fe2O3 grown on the sheets of RGO. Single crystalline structure feature of an α-Fe2O3 particle on the sheet of RGO by shown by the TEM images. The higher photocatalytic activity was observed for the composite of Fe2O3/RGO as compared to pristine α-Fe2O3 nanoparticles towards water oxidation. Reduced graphene Cuprous Oxide (Cu2O/RGO) photocatalysts were prepared by Niu, et al. by utilizing a similar procedure. Microspheres of RGO/TiO2 were obtained by a nonhydrolytic sol-gel reaction of acetone and Tetra Butyl Orth Titanate (TBOT) followed by the hydrothermal method. TBOT was added in excess acetone to prepare the pretreated titanium dioxide. 120 degrees to 180 degrees temperature was employed for the preparation of TiO2/RGO microspheres by hydrothermal method.
NH3 was employed as a medium solution during the process. It was observed that the pre-treated titanium dioxide has a diameter in the range of 1 μm and 2 μm and has a spherical shape. Microspheres with a collection of nanoflakes were obtained on the surface after treated at 120℃. Rough microspheres with nanorods aggregates were achieved when the temperature of the hydrothermal technique increase from 150℃ to 160℃.
The solvothermal technique was utilized for the preparation of Bi2WO6/RGO (BWO/RGO) composites. The Teflon lined autoclave was used for sealing the mixture of graphene oxide, Na2WO4.2H2O, and Bi(NO3)3.5H2O and maintained at 180℃. Finally, Bi2WO6 NPs were deposited on the sheets of RGO. The solvothermal method is extensively utilized for the fabrication of photocatalysts based on graphene.
Electrochemical and electrophoretic deposition: Graphene nanocomposites are synthesized by the electrochemical and electrophoretic deposition technique. Materials based on thin films are prepared by this technique. For example, nanostructures of zinc oxide, Cu2O, MnO2, and ZrO2 have been effectively deposited on layers of RGO. Generally, a steady current is initially applied to seed NPs (such as zinc oxide) on RGO, followed by the development of NPs under the stable potential means. The morphology of the sample is determined by the conductivity of RGO. Reduced graphene oxide with high conductivity is responsible for the growth of high quality hexagonal zinc oxide nanorods. Furthermore, mesoporous silica film is used for the synthesis of ordered nanostructures. The pores of percolated silica film are used for the deposition of CdSe nanocrystals on the face of graphene.
Materials and Methods
Sonochemical method: As mentioned above, the particle is more than numerous hundred nanometers in size on GR sheets by utilizing in situ developed approach. Two important problems must be well thought out for the plan and preparation of photocatalysts based on graphene.
• Control of particle size.
• Interface among the catalysts and graphene.
It is a great challenge that how to manage the size of the particle and enhance the interaction among the semiconductor and graphene. In the previous study, the ultrasonic waves are efficient to resolve the problem. Successful incorporation of titanium NPs onto layers of graphene was achieved in few hours by using ultrasonication. The condensation and pyrolysis of the dissolved titanium tetrachloride into titanium dioxide by ultrasonic waves results in the controlled size of NPs around 4 nm-5 nm on the sheets without the use of any surfactant. The SEM and TEM images established the uniform dispersion of titanium dioxide NPs on both the graphene surface and the interlayers. From the results, it is confirmed that ultrasound is very successful in dispersing NPs of titanium dioxide on layers of graphene.
The sonochemical technique was employed for the preparation of composite (WO3/GR) consisting of WO3 NPs and graphene sheets. The normal size of the particle of the WO3 NP was proscribed at around 12 nm on the graphene sheets without utilizing any surfactant.
The composite consisted of WO3 NPs and the 2D graphene sheet is a potential photocatalyst for the creation of oxygen. The synergistic property of chemically bonded graphene and WO3 results in the enhanced performance. The visible light absorption property of WO3/GR is improved by the sensitization of WO3 by graphene. The recombination of the e- -h+ couples generated by a photon is decreased by the chemical bonding of graphene and WO3 and results in enhanced photoconversion efficiency. A similar procedure is employed for the preparation of BWO on the surface of graphene sheets. There is improved performance in O2 production from water due to the mixture of the functionality of BWO with the sole characteristics of graphene.
Photo assisted method: Layer by layer congregation, template supported approach, etc. are different techniques that are developed to prepare graphene based photocatalysts.
Chemical vapor deposition: This technique is the competent method for uniformly growing the semiconductor metal oxides on graphene substrates. Vapor phase epitaxy is used by Kim, et al., for growing the nanoneedles of zinc oxide on graphene. Higher vertical alignments were observed due to the increase in the nucleation of zinc oxide nanoneedle at step edges and kinks on the surface of graphene than those grown on SiO2/Si.
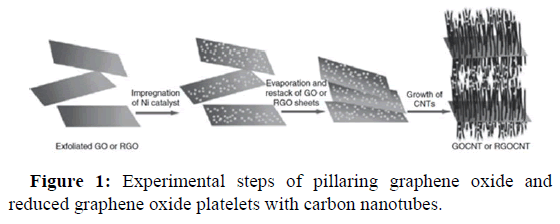
The CVD method was used by Zhang, et al., in which chemically exfoliated RGO was pillared with CNTs. Figure 1 shows that the solution of Ni(NO3)2 was mixed into chemically exfoliated graphene oxide or reduced graphene oxide platelets in water. Nickel including GO and reduced graphene oxide platelets were achieved by drying at 60℃ and were utilized as the catalyst in the CVD technique to grow carbon nanotubes with acetonitrile as the source of carbon. A robust three dimensional porous structure was formed by this composite material with reduced graphene oxide layers pillared by carbon nanotubes. On varying, the CVD time and the catalyst loading, the growth of 1-D carbon nanotubes can be restricted. Brilliant photocatalytic performance derived by visible light was exhibited by the CNT pillared reduced graphene oxide composite materials for the degradation of rhodamine B. Composite materials are excellent photocatalyst due to effective photosensitized electron addition, decreased electron radical recombination, and high ability of adsorption.
Graphene based photocatalytic composites
TiO2/graphene photocatalysts: The good stability, high efficiency, and low cost of titanium dioxide make it a dominating photocatalyst. Improved photocatalytic activities of titanium dioxide/graphene catalyst result in them as one of the most potential applicants for photocatalytic function.
Coupling of graphene and P25: The combination of graphene and P25 has been extensively studied, with an evident improvement of photocatalytic function. The one-step hydrothermal reaction was used for the synthesis of chemically bonded nanocomposites of P25/ graphene, with Ca 1 weight percentage of graphene content. Because of the sharing of -COOH groups on graphene oxide, P25 NPs dispersed on the carbon support and had an affinity to collect beside edges and wrinkles. Considerable enhancement in comparison to P25 was shown by a composite of P25/graphene in comparison to P25 for the methylene blue photodegradation. P25-RGO composites were synthesized by Fan, et al., by hydrothermal technique, hydrazine reduction, and UV assisted photo reduction technique. P25 reduced graphene oxide hydrothermal >P25 reduced GO photo reduction >P25 reduced GO hydrazine are the orders for the different photocatalytic activities exhibited by composites of P25/reduced GO for hydrogen evolution. Composite of P25 carbon nanotube was synthesized by the same method for comparison. It was observed from the study that the composite of P25 reduced GO is more efficient than the composite of P25 carbon nanotubes for the hydrogen evolution. Zhang and coworkers investigated the dissimilarity among P25/graphene and P25/carbon nanotubes composite photocatalysts for the duration of liquid phase degradation of dyes and gas phase degradation of benzene. For improving the photocatalytic activity of titanium dioxide, it was established that titanium dioxide/graphene was in essence like another titanium dioxide/carbon composite material.
The complexity of graphene based photocatalysts is specified by the conflicting results. Charge transfer and interfacial contact between graphene and nanoparticles, electrical property of graphene are the various factors that affect the integrated photocatalytic property of graphene based composites. The coupling of graphene with photocatalysts is demanding but enviable. The distinctive 2 dimensional structure of graphene sheets reimbursement the effective anchoring of semiconductor photocatalysts on their surface. The enhanced interfacial contact makes it a superior alternative for catalytic support.
Development of titanium dioxide on graphene: One pot and in situ routes are used to prepare photocatalysts with considerable efficiency by devoting various groups to the growth of titanium dioxide nanostructures on graphene. Liang and coworkers studied the effect of consistent coating and a strong combination of titanium dioxide and graphene oxide on Rhodamine B degradation (RhB). The result of interfacial stress on the property of titanium dioxide/graphene oxide composites was investigated by Chen, et al. The concentration of titanium dioxide is responsible for tuning the development of n-type and p-type semiconductors. P/n heterojunction could be observed when graphene oxide produced a p-type semiconductor. Semiconductors prepared by graphene oxide on the surface of photocatalysts could work as a sensitizer and improve their photocatalytic act. The effect of calcination atmosphere and graphene content on the photocatalytic action has also been investigated. Titanium dioxide/graphene composites showed improved performance as compared to P25 for the evolution of hydrogen. Higher activities were observed for the samples which are calcinated in the N2 atmosphere as compared to those which are calcinated in the air.
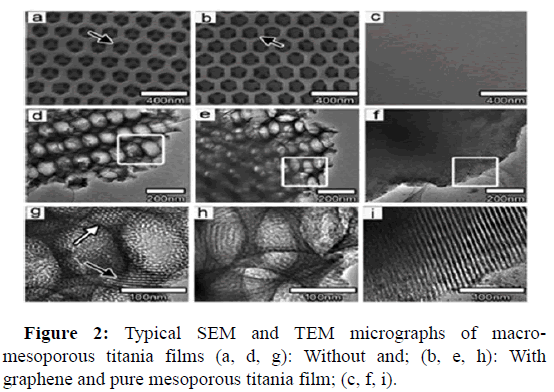
TiO2/graphene composite films: Photocatalytic films due to several features such as restoring, fixing, and recycling are the best candidates for photocatalytic applications. Graphene oxide coated with titanium dioxide exhibits improved efficiency of the photodegradation. It was associated with the proficient separation of charge and transportation among the planar structure and giant p-conjugation. A confined self-assembly technique was used by Du and coworkers to couple hierarchically ordered macro-mesoporous titania films to graphene. Figure 2 represents the SEM and TEM images of macro mesoporous titania films. Reduction in the length of the mesopore channel improved the available surface area within the thin layer and improve the mass transport through the layer is enhanced by the existence of interconnected macrospores in mesoporous films.
The obvious rate constants were found to be 11 times and 17 times higher for macro-mesoporous layers without and with graphene as compared to pure mesoporous titania films.
Results and Discussion
Metal oxide/G and metal sulfide/G photocatalysts
Various non-titanium dioxide based photocatalysts have been prepared. It has been observed from the study that several metal oxide compounds exhibited comparable photocatalytic ability, like Fe2O3, WO3, SnO2, etc. Zinc oxide is well thought out as an option to titanium dioxide for photocatalytic applications. Zinc NPs are combined with graphene oxide by in situ growth technique and on chemical reduction, this is converted into zinc oxide/graphene nano architectures. 4 times’ improvement of photocatalytic activity is achieved by Xu, et al., on using 2 wt. % graphene content as compared to pristine zinc oxide. The one pot reaction is carried out by utilizing sodium acrylate and results in situ development of Fe3O4 on graphene.
These composites show appropriate efficiency of removal and a fast separation from an aqueous medium using an external magnetic field.
Microwave irradiation technique was employed for the synthesis of nanocomposites of zinc sulfide/graphene. Thioacetamide was used utilized as a reducing agent and as a source of sulfur. Decoration of graphene with a well-known photocatalyst driven by visible light was also investigated. 1.0 wt. % is the optimal weight % of graphene in the nanocomposites of CdS clusters/G and leads to a 1.12 mmol h-1 photocatalytic hydrogen production rate. 22.5% is the resultant apparent quantum efficiency at a wavelength of 420 nm.
Metallate/graphene photo catalysts
Lately, attention has been devoted to the photocatalytic applications of metallates. The mixture of graphene with BiWO6 is considered the most significant photo catalyst among the Bi3+ based oxides. There is a shift of the fermi level and reduction in the CB potential due to charge equilibration and electronic interaction among Bi2WO6 and graphene. High movement effectiveness of photo induced e- and a negative shift in the Fermi level resulted from the improved photocatalytic activity of BiWO6/graphene nanoarchitectures. Outstanding 10 fold improvement was observed during the reaction of photoelectrochemical water splitting after the incorporation of graphene with BiVO4. Likewise, the photocatalytic performance of c- Bi2MoO6 has enhanced 4 folds after their incorporation with 1% graphene.
Magnetically separable nanocomposite photocatalyst of ZnFe2O4/ graphene was prepared by Fu and coworkers. Photoelectrochemical degrader and generation of hydroxyl radicals by photoelectrochemical decomposition of hydrogen peroxide and are the dual functions of photocatalysts. The photocatalytic activities of nanocomposites of InNbO4/graphene were reported by Zhang, et al. 1.87 times and 2.1 times are the kinetic constants of methylene blue and 2,4- dichlorophenol elimination by InNbO4/G.
Other G based photo catalysts
The combination of graphene with various other NMs has been extensively studied. Plasmonic photo catalysts with outstanding stability and better photocatalytic activity driven by visible light based on nanocomposites of Ag/AgX (X=Br, Cl)/GO were synthesized by Zhu and coworkers. In another study, graphene oxide and its reduced form were pillared with CNTs in the CVD technique by utilizing acetonitrile as a source of carbon. Exceptional property of electron transfer and exclusive porous structure results in the outstanding visible-light activity of the composite of carbon nanotube pillared reduced graphene oxide. The consequence of alteration of reduced graphene oxide with crystalline copper species was studied by Xiong, et al. The excited e- is passed from the reduced graphene oxide to the adsorbed oxygen as the copper species acts as an e- relay. The led to the degradation of RhB was degraded by continuously generated reactive oxygen species under irradiation of visible light.
Application of G based photo catalysts
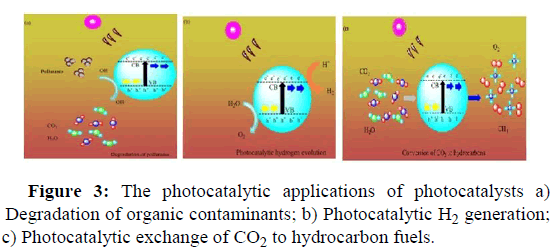
Graphene based photo catalysts play a vital function in resolving various ecological and contamination challenges. As given in Figure 3, there are three areas in which the photocatalysts based on graphene show considerable photocatalytic applications.
• Environmentally friendly chemical species resulted from the
degradation of organic pollutants.
• Photocatalytic change of carbon dioxide to hydrocarbon fuels.
• Photocatalytic hydrogen generation where there is a change of solar
energy into a hydrogen fuel.
Photodegradation of organic contaminants: Different kinds of intermediates and radicals are generated after photoexcitation.
Hydroxyl radicals are capable to hit the molecules around the surface of photocatalysts and are considered a powerful oxidizing agent. The advantage of photocatalysts has been extensively studied in the damage of both organic and inorganic substances. Photodegradation of organic substances by utilizing photocatalysts based on graphene is shown in Table 1.
| Type of catalysts | Graphene content | Contaminants | Results |
|---|---|---|---|
| P25/G | 1% | MB | 20% higher than P25-CNTs |
| P25/GO | 8.2 wt. (%) | MB | Apparent rate constant enhanced by a factor of 8.52 than P25. |
| P25/G | 10% | MB | 70% degraded after 5 h, compared to 10% of P25. |
| P25/G | 0.50% | Benzene | Alteration maintained at 6.4%. For P25 is reduced from 5.8% to 1.2% after 28 h. |
| TiO2/ G | 15 wt. (%) | RhB | The rate constant of SnO2 and TiO2 is 2.2 and 1.2 times larger than P25. |
| TiO2/G | 75% | MB | The k value was 2.5 times larger in comparison to P25. |
| TiO2/G | 30 mg | MB | 75% in 3 h, enhanced in comparison to P25. |
| TiO2/G | - | MO | Much larger than P25/G. |
| TiO2/GO | 0.14% | MO | Higher than P25. |
| TiO2/G | 10 wt. (%) | RhB | The rate constant was 3 times greater than P25. |
| TiO2/GO | 4.60% | MO | The degradation rate of MO was 7.4 times greater than P25. |
| G/TiO2/MCM-41 | 0.15 wt. (%) | 2-propanol | Improvement in the photocatalytic activity after coating of graphene. |
| TiO2 nanorods/GO | 40% TiO2 | C.I. acid orange | Higher effectiveness was exhibited by nanorods of TiO2 as compared to TiO2 NPs. |
| TiO2 nanorods/ GO | 30% | MB | Considerable improvement was attained in comparison to P25. |
| TiO2/GO | 0.03 mg | MB | TiO2/GO demonstrate enhancement in comparison to TiO2 film. |
| TiO2/G film | - | 2,4-dichlorophenoxyacetic Acid | The rate constant was 4 times greater than TiO2 film. |
| Macro-mesoporous TiO2/graphene | 0.6 wt. (%) | MB | The rate constant was 1.6 times greater than macro-mesoporous TiO2. |
| ZnO/G | - | Rh B | The rate of degradation was outstandingly improved |
| ZnO/G | 2 wt. (%) | MB | The activity was improved by about 4 times than ZnO. |
| ZnS/G | - | MB | ZnS/G had outstanding photocatalytic activity. |
| Fe3O4/G | - | Rh B, MG | 91% RhB and 94% MG were removed. |
| Au/G | - | Rh B | The rate constant was 1.8 times in comparison to P25. |
| Bi2WO6/G | - | Rh B | The activity was 3 times higher than the Bi2WO6 sample. |
| c-Bi2MoO6/G | 1 wt. (%) | MB | Enhancement inactivity by 4 times after 1.0 wt. % of graphene loaded. |
| ZnFe2O4/G | 20 wt. (%) | MB | Improvement in the activity after the addition of H2O2, 88% MB degradation was achieved in 5 min. |
| InNbO4/G | 3 wt. (%) | MB | The kinetic constant was 1.87 times higher in comparison to InNbO4. |
| CNTs/G | - | Rh B | 4 times faster in comparison to P25. |
| Copper modified G | 10 wt. (%) | Rh B | 3 times faster in comparison to P25. |
Table 1: New information on the utilization of G based photo catalysts for degrading selected organic contaminants.
Graphene based composites have been widely investigated for the photodegradation of cationic dye MB. The photocatalytic action of a composite of titanium dioxide/graphene is considerably improved under both irradiations of ultra violet and visible light. Various techniques have been utilized to explain the possible photocatalytic mechanism for the enhancement. Reduced graphene oxide wrapped titanium dioxide hybrid was prepared by one step photocatalytic reduction by Liu and coworkers. The transient photo voltage method was utilized by Wang, et al., for studying the photo induced charge transport between graphene and titanium dioxide. The mean lifetime of e--h+ couples was extended from ~10-7 s to ~10-5 s after their integration with graphene. Therefore, the double function of G in the composite was enhanced by:
• Increasing the e--h+ pair partition through the e- injection from
the conduction band of titanium dioxide into G.
• Significantly delaying the recombination of e--h+ pairs in the
excited TiO.
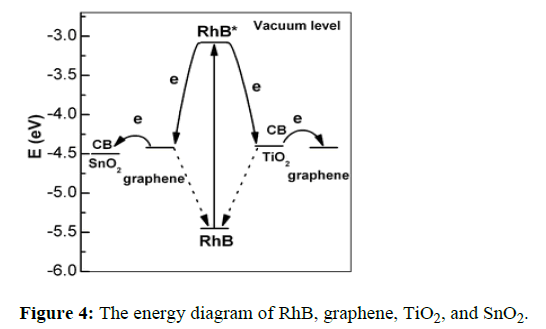
Photodegradation of RhB by using composites of graphene results in high photocatalytic activities. The transfer of e- from RhB* to the G semiconductor was thermodynamically favorable and a lot more viable than titanium dioxide. In Figure 4, diverse photocatalytic activities of titanium dioxide and SnO2 were explained by the different e- transport pathways and photosensitization method. Graphene acted as an e- intermediary due to the effective injection of e- from Rodamine B to graphene and it makes possible the transfer of an electron from RhB* to SnO2. The recombination process is retarded, and the photocatalytic activity is enhanced by this spatially separated electron and RhB+.
Graphene based photo catalysts also show potential applications in the degradation of different organic substances. A homogeneous titanium dioxide/graphene thin layer was prepared by Ng, et al., through the deposition of suspension of titanium dioxide/graphene. There is 4 times rise in the speed of photocatalytic degradation during the degradation of 2, 4-dichlorophenoxyacetic acid by titanium dioxide/graphene films. Nanocomposites of Pt/graphene/titanium dioxide were used for the degradation of DBS (Dodecyl Benzenesulfonate) photo catalytically. There is an increase in the mineralization of DBS by 3 times in comparison to P25. The improved photocatalytic degradation of organic molecules is given below;
• The adsorption of photocatalysts is improved by the interactions of
aromatic regions of graphene with organic molecule.
• Charge recombination is concealed by the movement of excited e
from titanium dioxide to graphene.
• Photo responding range is extended and the bandgap of titanium
dioxide is narrowed by the formation of Ti-O-C chemical bond.
H2 evolution from water photocatalytic splitting: It is an effective method due to no emission of CO2 and does not depend on the consumption of fossil fuels. Extended light absorption and inhibited recombination of photo induced carriers are significant considerations in this method. Graphene based material shows a potential application for hydrogen evolution from splitting water. Their electronic properties could be tuned by controlling the oxidation level. As a result, graphene oxide gradually catalyzed hydrogen generation under ultra violet or visible light irradiation from a 20 vol % aq. methanol solution.
Photocatalysts are combined with graphene due to the superb electrical property of graphene and hence lead to the development of their conductivity and activity of H2 production. For example, the H2 evolution rate of 20 mmol to 21 mmol was exhibited by titanium dioxide/reduced graphene oxide composites prepared by hydrothermal technique. That value was much superior to the as synthesized titanium dioxide NPs and the composites of titanium dioxide/carbon nanotube composites. Metal free graphite carbon nitride acts as a photocatalyst for water splitting. Recently, the combination of graphene with this polymeric photocatalyst has also been investigated.
Other application of G based photo catalysts: Solar energy conversion, environmental remediation, carbon dioxide reduction, chemical synthesis, and antibacterial applications are the various areas in which graphene enhanced photo catalysts are used. The effect of structural defects of G on the photocatalytic reduction of carbon dioxide to produce solar fuel was investigated by Liang and coworkers. Oxidation-reduction and solvent exfoliation are the two main solution based pathways by which graphene with dissimilar defect densities was synthesized. Larger enhancement in the photoreduction of carbon dioxide was exhibited by P25/G nanocomposites based on the less defective solvent exfoliated G. The high photocatalytic activity was observed for nanoparticles of titanium dioxide assembled on the nanosheets of graphene oxide for the photo reductive change of hexavalent chromium. The speed of conversion is found to be 5.4 times in comparison to P25. The effect of a G covering on the antibacterial activity of the titanium dioxide thin layer was investigated by Akhavan, et al. There is the enhancement of the antibacterial activity by 7.5 due to photocatalytic reduction of graphene oxide platelets for 4 hours.
Conclusion
Reactive OH radicals which are photo catalytically generated can act as sharp chemical scissors for photocatalytic production of G for electronics. Several photochemical tailoring of graphene, together with arbitrary patterning, film by film thinning, and ribbon cutting on any substrate are achieved by Zhang, et al., by using patterned titanium dioxide photomask. Carbon field effect transistor arrays are prepared by patterned graphene.
Water disinfection: Ag+ contamination occurs due to conventional water disinfection photocatalysts which are generally Ag based materials. The photocatalysts are protected from photo corrosion by graphene wrapping. Sulfonated GO/ZnO/Ag, GO/CdS, and GO/ TiO2/Ag are the several photocatalyst composites whose synthesis is demonstrated by Sun's group. Improved efficiency and photo stability were shown by both composites under visible light in the inactivation of Escherichia coli as compared to their corresponding part without adding graphene oxide. Metallic secondary contamination was avoided by metal free photo catalyst.
Photocatalysts based on Bi are extensively used to combine with graphene for water disinfection. Considerably higher efficiency was given away by Bi2MoO6/RGO nanocomposite in the inactivation of E. coli K-12 as compared to Bi2MoO6. From the studies, it was observed that toxic effects were exhibited by RGO on E. coli K-12.
Photodegradation of organic dyes: A high performance photo catalyst was formed by the combination of P25 with graphene under both UV and visible light. Higher activity was shown by the composite in the photodegradation of MB in comparison to both pure P25 and a P25/carbon nanotube composite. Efficient separation of charge improved adsorption and extended light absorption range resulted from the addition of graphene. In2S3 nanosheets/G composites driven by visible light were prepared by the hydrothermal method. WO3 nanorods/G was prepared for high efficient visible light driven photo catalysis.
References
- Lee C, Wei X, Kysar J W, Hone J (2008) Measurement of the elastic properties and intrinsic strength of monolayer graphene. Sci 321:385-388.
[Crossref] [Google Scholar] [PubMed]
- Novoselov KS, Geim AK, Morozov SV, Jiang DE, Zhang Y, et al. (2004) Electric field effect in atomically thin carbon films. Sci 306:666-669.
[Crossref] [Google Scholar] [PubMed]
- Lightcap IV, Kamat PV (2013) Graphitic design: Prospects of graphene based nanocomposites for solar energy conversion, storage and sensing. Acc Chem Res 46:2235-2243.
[Crossref] [Google Scholar] [PubMed]
- Zhu J, Holmen A, Chen D (2013) Carbon nanomaterials in catalysis: Proton affinity, chemical and electronic properties, and their catalytic consequences. Chem Cat Chem 5:378-401.
- Machado BF, Serp P (2012) Graphene based materials for catalysis. Catal Sci Technol 2:54-75.
- Wu S, He Q, Zhou C, Qi X, Huang X, et al. (2012) Synthesis of Fe3O4 and Pt nanoparticles on reduced graphene oxide and their use as a recyclable catalyst. Nanoscale 4:2478-2483.
[Crossref] [Google Scholar] [PubMed]
- Wang QH, Kalantar-Zadeh K, Kis A, Coleman JN, Strano MS (2012) Electronics and optoelectronics of two dimensional transition metal dichalcogenides. Nat Nanotechnol 7:699-712.
[Crossref] [Google Scholar] [PubMed]
- Osada M, Sasaki T (2012) Two dimensional dielectric nano sheets: Novel nano electronics from nanocrystal building blocks. Adv Mater 24:210-228.
[Crossref] [Google Scholar] [PubMed]
- Hirsch A, Englert JM, Hauke F (2013) Wet chemical functionalization of graphene. Acc Chem Res 46:87-96.
[Crossref] [Google Scholar] [PubMed]
- Dubois SM, Zanolli Z, Declerck X, Charlier JC (2009) Electronic properties and quantum transport in graphene based nanostructures. Eur Phys J B 72:1-24.
- Dragoman M, Dragoman D (2009) Graphene based quantum electronics. Prog Quantum Electron 33:165-214.
- Zhang Y, Nayak TR, Hong H, Cai W (2012) Graphene: A versatile nano platform for biomedical applications. Nanoscale 4:3833-3842.
[Crossref] [Google Scholar] [PubMed]
- Huang Y, Liang J, Chen Y (2012) An overview of the applications of graphene based materials in supercapacitors. Small 8:1805-1834.
[Crossref] [Google Scholar] [PubMed]
- Jiang H, Lee PS, Li C (2013) 3D carbon based nanostructures for advanced supercapacitors. Energy Environ Sci 6:41-53.
- Dong L, Chen Z, Yang D, Lu H (2013) Hierarchically structured graphene based supercapacitor electrodes. Rsc Advances 3:21183-21191.
- Cao X, Shi Y, Shi W, Lu G, Huang X, et al. (2011) Preparation of novel 3D graphene networks for supercapacitor applications. Small 7:3163-3168.
[Crossref] [Google Scholar] [PubMed]
- Wang Z, Zhou X, Zhang J, Boey F, Zhang H (2009) Direct electrochemical reduction of single layer graphene oxide and subsequent functionalization with glucose oxidase. J Phys Chem A 113:14071-14075.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi