Case Report, J Genit Syst Disor S Vol: 0 Issue: 0
Malignant Spermatic Cord Tumor as a Rare Scrotal Lesion: Case Report and Literature Review
Draeger DL1*, Erbersdobler A2, Protzel C1 and Hakenberg O1
1Department of Urology, University of Rostock, Ernst-Heydemann-Strasse 6, 18057 Rostock, Germany
2Institute of Pathology, University Rostock, Germany
Corresponding author : Désirée Louise Dräger, MD
Dept of Urology, University of Rostock, Ernst-Heydemann-Strasse 6, 18057 Rostock, Germany
Tel: 0381 494 7819
E-mail: Desiree-Louise.Draeger@med.uni-rostock.de
Received: May 09, 2016 Accepted: July 13, 2016 Published: July 20, 2016
Citation: Draeger DL, Erbersdobler A, Protzel C, Hakenberg O (2016) Malignant Spermatic Cord Tumor as a Rare Scrotal Lesion: Case Report and Literature Review. J Genit Syst Disor S2. doi:10.4172/2325-9728.S2-004
Abstract
Malignant Spermatic Cord Tumor as a Rare Scrotal Lesion: Case Report and Literature Review/strong>
Malignant tumors of the spermatic cord are urologic rarities. Due to their rarity the therapeutic management is often inconsistent and characterized by individual clinical decisions. The treatment of choice is inguinal orchiectomy with extensive resection of the surrounding soft tissue. These neoplasms occur mostly in elderly patients. We report two patients who presented with scrotal masses and review what is currently known about this entity.
Keywords: Paratesticular tumors; Spermatic cord tumors; Surgery; Radiotherapy; Chemotherapy
Keywords
Paratesticular tumors; Spermatic cord tumors; Surgery; Radiotherapy; Chemotherapy
Background
Malignant tumors of the spermatic cord are rare. They are mostly of mesenchymal origin treatment of choice is high inguinal orchiectomy with extensive resection of the surrounding soft tissue. They occur in elderly men (mean age at diagnosis around 65 years) and most commonly in Caucasians (86.7%). The localized tumors have a potential for (early) lymphatic metastasis (>29%). The histological findings are heterogeneous: liposarcoma (46.4%), leiomyosarcoma (20%), histiocytoma (13%) and rhabdomyosarcoma (9%). The rhabdomyosarcoma variant is more common in younger patients (mean age around 26 years).
Case Reports
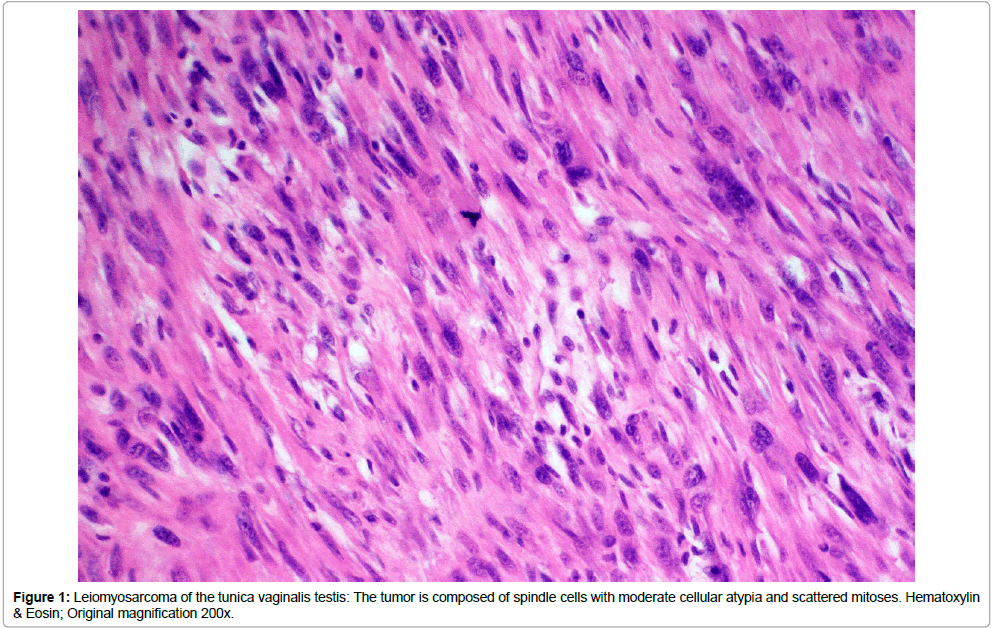
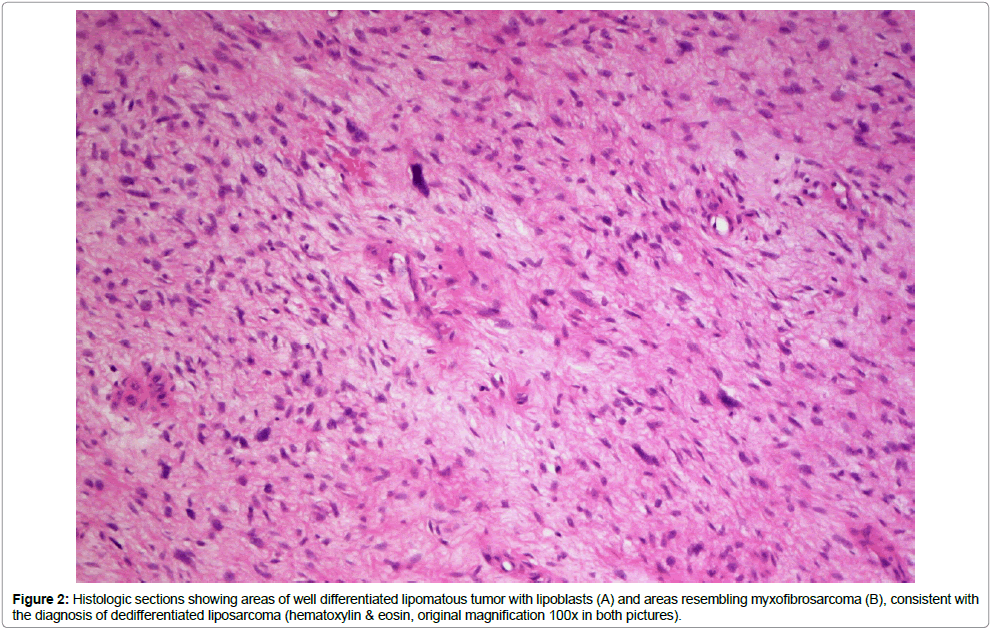
We report two patients (65 and 82 years old) who presented with an asymptomatic, paratesticular small mass for diagnosis and treatment. Both patients had a slowly enlarging painless paratesticular mass. On examination, both had a solid, indolent intrascrotal mass, which was clearly separated from the testis and epididymis. Otherwise physical examination was normal in both cases, as was blood chemistry including relevant tumor markers. Ultrasound showed an inhomogeneous tumor structure with normal testis and epididymis. Due to the growing nature of the lesions, surgical exploration was done in both cases and the lesions were removed with wide resection margins. Histology showed a liposarcoma (Figure 1) of the spermatic cord in the 82-year-old patient and a leiomyosarcoma (Figure 2) in the 65-year-old patient. Surgical margins were negative, the postoperative courses were uneventful and no adjuvant treatment was done. Both patients follow close follow-up due to the high tendency for recurrence.
Discussion
In the literature there 185 case reports of liposarcomas of the spermatic cord. A diagnosis based on clinical examination is impossible, palpation of a non-tender mass being the usual findings can [1-5]. Ultrasound should be done as the primary imaging modality clearly distinguishing the mass from the testis and epididymis; its sensitivity for differentiating intratesticular from extratesticular lesions is high (95-100%) [6]. The malignant character is suggested by the solid nature of the lesion and the history of slow enlargement. The differential diagnosis are cystic lesions (funiculozele, hydrocele) but ultrasound can exclude these [1,7-9]. Paratesticular sarcomas usually have a heterogeneous structure and may appear hypervascular if Doppler sonography is done [8,10]. Liposarcomas often have a heterogeneous appearance on ultrasound due to variable amounts of adipose tissue contained [8,10]. Leiomyosarcomas, in contrast, often appear as well-defined masses with a dense connective appearance which may, however, show intratumoral bleeding, necrosis or cystic degeneration [11]. Thus, although sonography is useful with respect to the definition of the extra-testicular origin of the mass, sonographic findings are often variable and unspecific. With regard to tumor size, delineation and pattern of echogenicity there are no clear sonographic diagnostic criteria.
Further imaging is therefore useful if such a tumor is suspected and MRI is most useful for scrotal pathology [5,8]. PET-CT (FDGPET) can identify dedifferentiated liposarcoma but this has not been established for clinical routine [12]. Likewise, although described, a role of fine needle aspiration cytology in the diagnosis of spermatic cord tumors is not clearly defined [13,14].
Since the spermatic cord is derived from the mesoderm, 90% of all neoplasms of the spermatic cord are sarcomas [15]. The first paratesticular sarcoma was described by Lesauvage in 1845. Overall, there are different histological variants: liposarcoma (46.4%), leiomyosarcoma (20%), histiocytoma (13%) and rhabdomyosarcoma (9%) [1,3].
Liposarcomas are most common. The WHO classifies five categories in order of increasing malignancy: well differentiated, dedifferentiated, myxoid, round-cell and pleomorphic. Most liposarcomas are well differentiated, low-grade disease without or only with minimal tendency to metastasize but they can be locally invasive [16]. Most patients acquire this disease in the fifth or sixth decade of life.
With 20%, leiomyosarcomas are the second most common entity, with a peak incidence in the 6th and 7th decade of life. The tumor is derived from the smooth muscle of the vas deferens, blood vessels or the cremaster muscle [17]. Local recurrence is common (50%), despite radical surgery [18,19]. This high rate of recurrence can be reduced by adjuvant radiation. The risk of relapse is related to tumor size, surgical margin status, inguinal location of the tumor, histological dedifferentiation and depth of invasion [1,2,20,21]. Local recurrence is occurs at the residual spermatic cord, the scrotum and the surrounding pelvic tissues, with or without lymph node involvement.
If the lesion occurs in the spermatic cord, a recurrence generally extends along the spermatic cord through the internal inguinal ring into the pelvis. Lymphatic metastases are usually in pelvic and/or para-aortic lymph nodes. Hematogenous spread occurs to the liver and lungs. Late recurrences in the form of distant metastasis are not uncommon, and have been described after 15 years in 42%. Thus, longterm follow-up is required. Patients should be monitored closely for a minimum of 36 months with further, less frequent regular follow-up for 15 years [1]. Follow-up should include chest X-rays and CT scans.
There is no standard treatment protocol as there is no consensus on standardized surgical or adjuvant treatment. Treatment of choice is radical surgical excision (inguinal orchiectomy) [3]. The extent of resection of the surrounding connective tissue is controversial [1]. Certainly a simple excision correlates with a high likelihood of recurrence because these tumors have a “pseudo-capsule” with penetration of tumor cells into the periphery. However, to the anatomical boundaries of the inguinal region a very extensive resection is usually not possible.
Regarding adjuvant treatment there are also no clear standards. The role of prophylactic lymphadenectomy is controversial. Although regularly performed in some centers, the true incidence of para-aortic and pelvic lymph node metastases is unknown [20]. A consensus exists regarding the indication for retroperitoneal lymphadenectomy (pelvic, inguinal and paraaortic) for liposarcoma and leiomyosarcoma of the spermatic cord although such extensive lymphadenectomy carries some morbidity. The role of adjuvant radiotherapy and chemotherapy is also under discussed [1,3]. Patients with high risk tumors and a high risk of recurrence will usually benefit from adjuvant radiotherapy. Also, the general use of prophylactic radiotherapy after surgical resection with negative surgical margins has been described to reduce the local recurrence rate. The rationale for this is that due to the anatomy of the region limiting very radical surgery, adjuvant radiotreatment should be recommended. The radiation field is the inguinal canal and the ipsilateral pelvis and scrotum [1,18,21]. Although this concept seems plausible there is no study large enough to support this strategy on an evidence basis [1]. There are also not enough data regarding the proper radiation dose [22]. The currently recommended dose is 60-65 Gy over 6 weeks [1,18]. For the use of radiotherapy as a neoadjuvant treatment, there is no evidence that this improves outcomes.
Systemic chemotherapy has no clear role except for the treatment of juvenile rhabdomyosarcoma [23]. Rhabdomyosarcomas are the only paratesticular tumors which show a significant response in all stages to combination chemotherapy with vincristine, dactinomycin and ifosfamide/cyclophosphamide.
The prognosis is as varied as the treatment is. Kyle (1966) reported a 5-year survival of 10-15% [24], but more recent data suggest a much better 5-year survival of approximately 50-80% which may be attributed to improved diagnosis and treatment. The prognosis depends on early diagnosis and treatment, as well as tumor stage and -grading.
Summary
Despite their rarity malignant spermatic cord tumors are an important differential diagnosis of scrotal masses. The majority of neoplasms of the spermatic cord are of mesenchymal origin, therefore sarcomas and about 30% are malignant. There are few clear treatment standards due to the rarity of the disease with evidence consisting of small series and case reports. Radical surgery with wide resection margins are the first treatment modality. Adjuvant radiotherapy is certainly needed for undifferentiated tumors and for recurrences but is probably beneficial for more cases. The role of extensive lymphadenectomy is controversial. Only rhabdomyosarcoma in young patients has been shown to benefit from multimodal treatment with surgery, adjuvant radiotherapy and multimodal chemotherapy. Due to the high risk of recurrence close and long-term follow-up is necessary.
References
- BalloMT, Zagars GK, Pisters PW, Feig BW, Patel SR, et al. (2001) Spermatic cord sarcoma: outcome, patterns of failure and management. J Urol 166:1306-1310.
- Froehner M, Koch R, Lossnitzer A, Schober RR, Schuler M, et al. (2014) Adult inguinoscrotal sarcomas: outcome analysis of 21 cases, systematic review of the literature and meta-analysis.World J Urol32:445-451.
- Coleman J, Brennan MF, Alektiar K, Russo P (2003) Adult sper matic cord sarcomas: management and results. Ann SurgOncol 10:669-675.
- Montgomery E, Fisher C (2003) Paratesticularliposarcoma: a clinicopathologic study. Am J SurgPathol 27:40-47.
- Woodward PJ, Schwab CM, Sesterhenn IA (2003) From the archives of the AFIP: extratesticular scrotal masses: radiologic-pathologic correlation.Radiographics 23:215-240.
- Frates MC, Benson CB, DiSalvo DN, Brown DL, Laing FC, et al. (1997) Solid extratesticular masses evaluated with sonography: pathologic correlation. Radiology. 204:43-46.
- Chintamani, Tandon M, Khandelwal R, Jain S, Narayan N, et al. (2010) Liposarcoma of the spermatic cord: a diagnostic dilemma. JRSM Short Rep 1:49.
- Akbar SA,Sayyed TA, Jafri SZ, Hasteh F, Neill JS. (2003) Multimodality imaging of paratesticular neoplasms and their rare mimics. Radiographics 23:1461-1476.
- Blaivas M, Brannam L (2004) Testicular ultrasound. Emerg Med Clin North Am 22:723-748.
- Secil M, Kefi A, Gulbahar F, Aslan G, Tuna B, et al. (2004) Sonographic features of spermatic cord leiomyosarcoma. J Ultrasound Med 23:973-976.
- Kyratzi I, Lolis E, Antypa E, Lianou MA, Exarhos D (2011) Imaging features of a huge spermatic cord leiomyosarcoma: Review of the literature. World J Radiol 3:114-119.
- Lipset RE, Kirpekar M, Cooke KS, Abiri MM (1997) US case of the day. Myxoidliposarcoma of the spermatic cord.Radiographics 17:1316-1318.
- Dalla Palma P, Barbazza R (1990) Well-differentiated liposarcoma of the paratesticular area: report of a case with fine-needle aspiration preoperative diagnosis and review of the literature. DiagnCytopathol 6:421-426.
- Bosch-PrÃncep R, MartÃnez-González S, Alvaro-Naranjo T, Castellano-MegÃas VM, Salvadó-Usach MT, et al. (2000) Fine needle aspiration and touch imprint cytology of a malignant fibrous histiocytoma of the spermatic cord. Case report. ActaCytol 44:423-428.
- Arlen M, Grabstald H, Whitmore WF Jr. (1969) Malignant tumors of the spermatic cord. Cancer 23:525-532.
- Schwartz SL, Swierzewski SJ 3rd, Sondak VK, Grossman HB (1995) Liposarcoma of the spermatic cord: report of 6 cases and review of the literature. J Urol 153:154-157.
- Avila PJ, Capell González M, MuniesaCalderó M, YelletischAraño A, BadÃaTorroella F, et al. (1993) Leiomyosarcoma of the spermatic cord. ActasUrolEsp 17:464-467.
- Fagundes MA, Zietman AL, Althausen AF, Coen JJ, Shipley WU (1996) The management of spermatic cord sarcoma.Cancer 77:1873-1876.
- Merimsky O, Terrier P, Bonvalot S, Le Pechoux C, Delord JP, et al. (1999) Spermatic cord sarcoma in adults.ActaOncol 38:635-638.
- Folpe AL, Weiss SW (2000) Paratesticular soft tissue neoplasms. Semin Diagn Pathol 17:307-318.
- Rabbani F, Wright JE, McLoughlin MG (1997) Sarcomas of the spermatic cord: significance of wide local excision.Can J Urol 4:366-376.
- Banowsky LH, Shultz GN (1970) Sarcoma of the spermatic cord and tunicas: review of the literature, case report and discussion of the role of retroperitoneal lymph node dissection.J Urol 103:628-631.
- Stewart RJ, Martelli H, Oberlin O, Rey A, Bouvet N, et al. (2003) Treatment of children with nonmetastaticparatesticularrhabdomyosarcoma: results of the Malignant Mesenchymal Tumors studies (MMT 84 and MMT 89) of the International Society of Pediatric Oncology. J ClinOncol 21:793-798.
- Kyle VN (1996) Leiomyosarcoma of the spermatic cord: a review of the literature and report of an additonal case. J Urol96:795-800.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi