Research Article, J Nanomater Mol Nanotechnol Vol: 7 Issue: 6
Novel Synthesis and Magnetic Properties of PVP Capped Cobalt Nanostructures
Dalavi SB1 Raja MM2 and Panda RN1*
1Department of Chemistry, Birla Institute of Technology and Science, Pilani, Goa, India
2Defense Metallurgical Research Laboratory, Hyderabad, India
*Corresponding Author : Rabi N Panda
Department of Chemistry, Birla Institute of Technology and Science, Pilani, Goa, India
Tel: +91 08322580311
E-mail: rnp@goa.bits-pilani.ac.in
Received: July 02, 2018 Accepted: July 26, 2018 Published: August 01, 2018
Citation: Dalavi SB, Raja MM, Panda RN (2018) Novel Synthesis and Magnetic Properties of PVP Capped Cobalt Nanostructures. J Nanomater Mol Nanotechnol 7:6. doi: 10.4172/2324-8777.1000257
Abstract
Novel polyol methodologies (named as: route-1 and route-2) have been developed for synthesis of PVP capped nanostructured Co in organic (DMF) medium at lower temperature of 110oC. Co nanoparticles (nps) crystallize in hcp and fcc structures with average crystallite size of 16.4 nm and 37.1 nm, for route-1 and route-2, respectively. TEM micrographs show chain-like and nearly spherical nanostructures for Co nps synthesized via route-1 and route-2, respectively. We could obtain the maximum value of MS and Hc, i.e., 167 emu/g and 357 Oe, respectively, for nanostructured Co synthesized via route-1 at 100 K. Variations in saturation magnetizations (Ms) and coercivity (Hc) values have been explained mainly on the basis of fine particle size, shape, surface and altered crystal anisotropies.
Keywords: Nanoparticles; X-ray techniques; Metal and alloys; Electron microscopy; Magnetic materials
Introduction
Several reports are available on Co and Fe based alloys which have potential high temperature applications in magnetic [1,2]. Recently, Co nanoparticles (NPs) with high magnetic moment have great applications in data storage, biomedical, and biosensor fields [2]. Although, fine particle size and surface effects are known to induce a reduction in Ms [3], development of polyol method which can be used for the synthesis of high moment Fe-Co NPs (273 emu/g) having a particle size of 100 nm has been achieved [4]. However, synthesis of high moment NPs with particle size below 100 nm is a challenging task. Effective modifications in polyol methodology in order to get desired magnetic properties may include control on particle growth, size, morphology and stability of the nanoparticles, majorly [5,6].
In this study, we have used PEG as reducing agent in organic solvent (DMF) for the synthesis of PVP capped Co based nanostructures at relatively low temperature of 110°C. The as synthesized materials were annealed in order to study structural and magnetic properties.
Experimental
Synthesis of nanostructured Co
In order to synthesize Co nanostructured materials, we have procured cobalt chloride hexahydrate (CoCl2·6H2O), and Polyethyleneglycol (PEG-200) Molychem, India; N,N dimethylformamide (DMF), sodium hydroxide (NaOH) from Fisher scientific, India; polyvinylpyrrolidone (PVP, Molecular weight, Mw=58000) from Acros organics, USA. The chemicals were of analytical grade and have been used without further purification.
In a typical synthesis, 0.476 g (2 mmol) CoCl2·6H2O and 20 mL of DMF were added into a 250 mL three neck flask. Then, PVP (1:1 wt. ratios with the metal salt) and PEG-200 (4 mL) were added with vigorous stirring at RT. The mixture was heated to 100°C-110°C for the duration of 5 min. To the resulting solution, NaOH pellets (2 pellets) were added. Reaction mixture changes from light pink to black indicating the formation of Co nanomaterials. The reaction mixture was maintained at 100°C-110°C for 15 min and then cooled down to RT. 100 mL acetone was added to precipitate solids. The colloid dispersion was separated by centrifugation at 5000 rpm. The resulting solid was washed with water repeatedly till the filtrate shows neutral pH, then with acetone and dried in vacuum at RT (as-prepared materials). The final products were obtained from as-prepared materials using two different drying and processing conditions, i.e., (i) route-1 and (ii) route-2 representing products without and with traces of NaCl, respectively. In route-1, solid obtained after centrifugation were washed with distilled water repeatedly (in order to wash out NaOH and NaCl) and finally with acetone. This sample were annealed at 600°C for 6 h duration under N2 (g) flow. In route-2, solid obtained after centrifugation were first annealed at 600°C for 6 h under high purity N2 (g) flow and then materials were washed using distilled water (in order to remove water soluble NaOH and NaCl compounds) followed by final washing with acetone.
Instrumental characterizations
Co NPs were characterized by using X-ray diffraction (XRD) method, transmission electron microscopy (TEM) and vibrating sample magnetometer (VSM). The XRD patterns were recorded using a powder X-ray Diffractometer (Model: Rigaku Mini Flex II, Japan) using Cu Kα radiation, (λ=1.5405 Ǻ) at scan speed of 3°/min. XRD crystallite size was calculated using Scherrer equation, t=0.9λ/ Bcosθ, where‘t’ is crystallite size, ‘λ’ is wavelength of X-ray radiation, B is full width at half maximum (FWHM) in radian and ‘θ’ is the diffraction angle. Particle size, shape and morphology of product were examined by TEM (Model: PHILIPS CM200, Oregon). The magnetic measurements were carried out on solid powder material using VSM (Model: ADE-EV9) which can provide a magnetic field up to 20 kOe.
Results and Discussion
XRD studies of Co nanoparticles
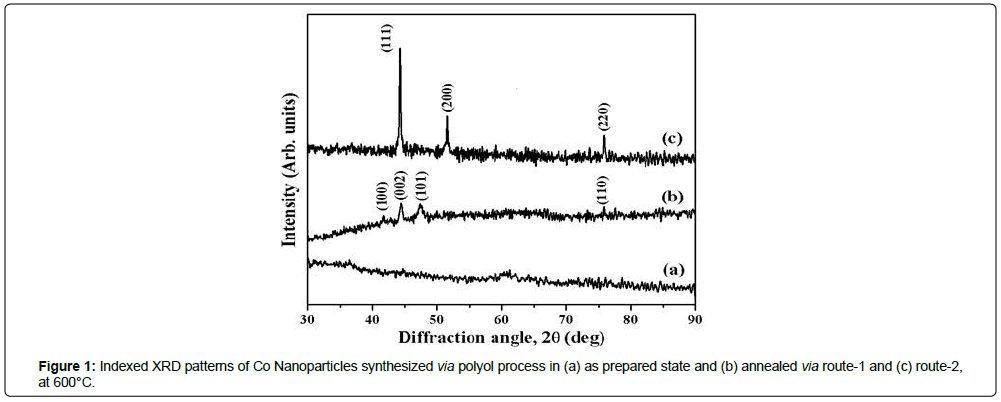
The XRD patterns of as-prepared and annealed Co nps at 600°C for 6 h under N2(g) atmosphere are presented in Figures 1a-1c. The absence of diffraction peaks in XRD patterns for as-prepared Co nanomaterials, except a very small intensity peak centered at 60°C indicates the amorphous nature of the materials (Figure 1a). However, Co (route-1) crystallizes in hcp phase (α) (JCPDS # 05-0727) whereas Co (route-2) in fcc phase (β) (JCPDS # 15-0806) after heat treatment (Figures 1b and 1c). One should note the enhancement of (002) peak intensity compared to that of (100) peak intensity in the observed XRD pattern for hcp Co. These results indicate the preferential growth of the crystal lattice along (002) planes along c-axis [7]. The estimated values of the lattice parameters for Co nanocrystals having hcp(α) phase are estimated to be 2.4972 Ǻ and 4.0734 Ǻ for ‘a’ and ‘c’, respectively. Also, for fcc (β) Co (route-2), the value of lattice parameters, a, is estimated to be 3.5403 Ǻ. Average crystallite sizes estimated from XRD line broadening for hcp Co (route-1) and fcc Co (route-2) were found to be 16.4 nm and 37.1 nm, respectively (Table 1).
In case of bulk, hcp phase of Co is stable below 450°C whereas stability of fcc phase is extended beyond 450°C. Therefore, the presence of hcp phase at 600°C in Co (route-1) nanostructured materials may be attributed to the fine particle nature of the material [8]. The observation of single fcc phase for Co (route-2) rather than energetically stable hcp phase may be due to annealing in presence of crystalline NaCl matrix. Such kind of stabilizations of fcc phase in a matrix has already been evident and reported for cobalt incorporated in crystalline copper matrix [9]. The observed values of the lattice parameters for hcp Co (i.e. a=2.4972 Ǻ and c=4.0734 Ǻ) and fcc Co (i.e. a=3.5403 Ǻ) phases are in well agreement with the reported values of lattice parameters, i.e. a=2.5088 Å, c=4.0762 Å for α-Co(hcp) and a=3.5448 Å for β-Co(bcc) [10].
TEM studies of Co nanoparticles
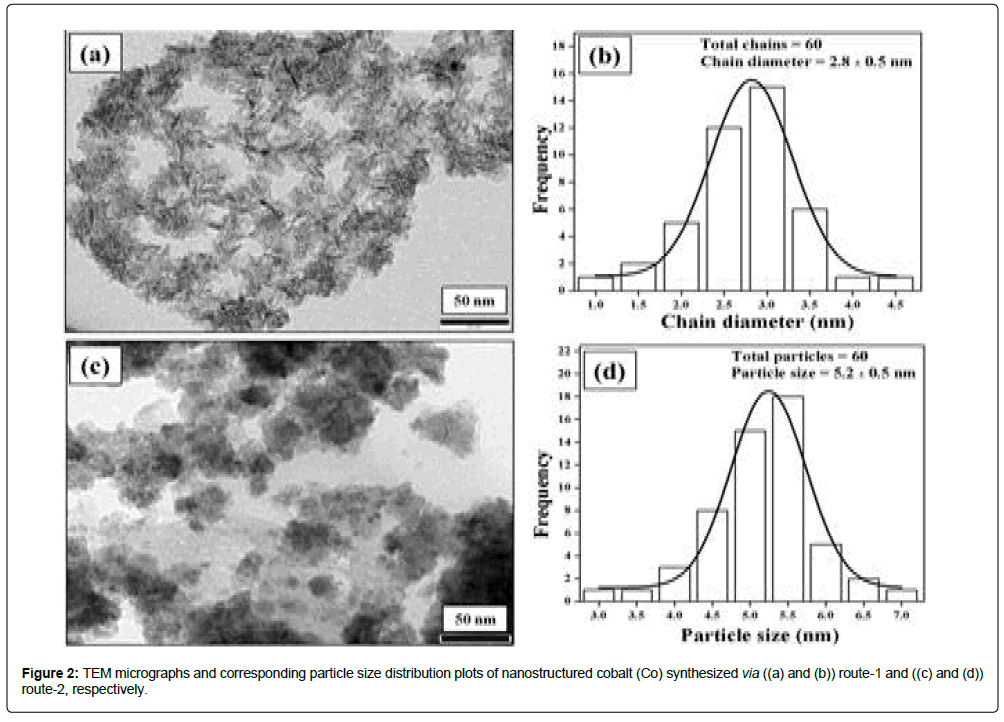
TEM micrographs and the corresponding TEM particle size distribution plots of Co nps products obtained via route-1 and route-2 are presented in Figures 2a-2d respectively. TEM morphologies and particle sizes are tabulated in Table 1. TEM micrograph of Co (route-1) shows chain-like nanostructures. The estimated value of the average chain diameter was found to be 2.8 ± 0.5 nm and chain lengths varies from 15-26 nm. TEM micrograph of Co (route-2) shows nanostructures with nearly spherical morphologies having TEM particle size of 5.2 ± 0.5 nm. The estimated TEM particle sizes are smaller than crystalline sizes, i.e. 16.4 nm and 37.1 nm for Co (route-1) and Co (route-2), respectively. The reason for such differences might be due to the fact that TEM particle sizes may correspond to a local probe of a region with smaller size particles only while average crystallite size corresponds to the mean value of crystallite sizes of an assembly of crystallites [11].
Magnetic properties of nanostructured Co
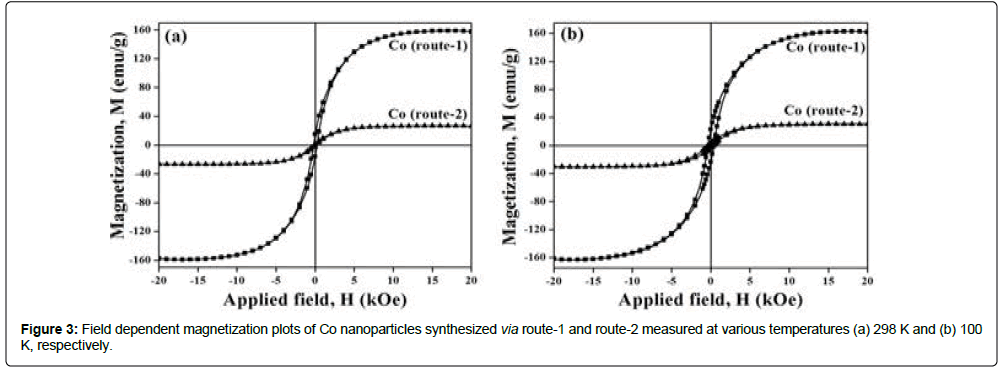
M vs. H curves for nanostructured Co (route-1 and route-2), measured at RT and at 100 K, are shown in Figure 3. The estimated values of MS and HC for Co samples are tabulated in Table 1. Co nps annealed at 600oC, contain coated organic materials at the surface. Total wt% of CHN content for Co (route-1) and Co (route-2) samples were found to be 0.1% and 10.2%, respectively. Therefore, two different values of MS are reported herewith, i.e., one that denotes MS without considering wt% of organic layer and the other, M′S, after wt% corrections corresponding to total CHN content in the material. The values of the magnetic parameters (MS, M′S and Hc) for Co (route-1) and Co (route-2) materials measured at 298 K and 100 K are tabulated in Table 1.
| Materials | Average Crystallite size (nm) | TEM Morphology | TEM Particle dimension (nm) | MS (emu/g) | M′S (emu/g) | HC (Oe) | |||
|---|---|---|---|---|---|---|---|---|---|
| RT | 100 K | RT | 100 K | RT | 100 K | ||||
| Co (route-1) |
16.4(1) | Chain | D=2.8 ± 0.5 L=15-26 |
162.5 | 166.9 | 162.7 | 167 | 220 | 357 |
| Co (route-2) |
37.1(1) | Spherical | D=5.2 ± 0.5 | 26.7 | 31.4 | 29.7 | 35 | 185 | 369 |
Table 1: XRD, TEM and Magnetic characteristics of nanostructured cobalt synthesized via route-1 and route-2. Ms, M′S and Hc values denote saturation magnetization, corrected Ms and coercivity, respectively.
M′S values of Co (route-1) measured at RT, i.e., 162.7 emu/g, is matching well with the value for bulk hcp Co (162 emu/g) whereas M′S value of Co (route-2) i.e., 29.7 emu/g, is found to be significantly less than the value reported for bulk fcc Co (172 emu/g) [12]. The observation of large reduction in M′S for nanocrystalline Co (route-2) sample may be attributed to several factors such as size, shape, spin canting at the surface, altered crystal anisotropies etc., [13]. Possibly, the occurrence of chain like structures in Co nps obtained via route-1 is responsible for shape induced larger crystal anisotropies and intergranular interactions. This explains the observation of high moment Co atoms leading to high value of saturation magnetization in Co (route-1) materials. The specific magnetizations for Co (route-2) materials measured at 100 K show enhancement of 0.06% and 3.55%, respectively, when compared to the values at RT and the obs ervation is in good agreement with the reported results [14].
It has been reported that hcp Co is magnetically hard phase (HC>400 Oe) whereas fcc Co is magnetically soft phase (HC<100 Oe) [15]. The HC value of hcp Co (route 1), i.e., 220 Oe at RT, is less than the literature reported value of 583 Oe for magnetically hard hcp Co phase with particle size range of 10-12 nm [16]. HC value of fcc Co (route-2) at RT, i.e., 185 Oe, is larger than the values expected for soft magnetic materials. These results of alteration of Hc values may have origin from several factors such as particle size, dimension and their distribution [3]. When the measure temperature is lowered down to 100 K, the values of HC for nanostructured cobalt synthesized via route-1 and route-2 are increased by factors of 1.6 and 2.0, respectively, compared to the HC values at RT.
Conclusion
Co nanoparticles have been successfully synthesized via modified polyol reduction route using PVP as capping agent via two different synthesis strategies, i.e., route-1 and route-2, at 110oC. Accordingly, two distinct nano-dimensional products i.e., hcp with chain structure (dimension ~ 20 × 2.8 nm) for route-1 and fcc with spherical shape (diameter 5.2 nm) were synthesized. The average crystallite sizes of 16.4 nm and 37.1 nm were estimated for Co nps synthesized via route-1 and route-2, respectively. Co nps synthesized via route-1 has large value of MS, i.e., 167 emu/g at 100 K compared to 35 emu/g for route-2 at 100 K. Similarly, different HC values have been observed for Co nps synthesized via different routes (357 Oe and 369 Oe for Co (route-1) and Co (route-2) at 100 K respectively). These alterations of magnetic parameters have been explained on the basis of size, shape, spin-canting and altered crystal anisotropies.
References
- Dmitrieva NV, Lukshina VA, Volkova EG, Potapov AP, Gaviko VS, et al. (2013) Fe- and Co-based nanocrystalline soft magnetic alloys modified with Hf, Mo, and Zr: magnetic properties, thermal stability, and structure. Alloys (Fe0.6Co0.4)86Hf7B6Cu1 and (Fe0.7Co0.3)88Hf7B4Cu1. Phys Met Metallogr 114: 129-137.
- Frey NA, Peng S, Cheng K, Sun S (2009) Magnetic nanoparticles: synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem Soc Rev 38: 2532-2542.
- Dalavi SB, Panda RN (2015) Magnetic properties of nanocrystalline Co and Ni synthesized via superhydride reduction route. J Magn Magn Mater 374: 411-416.
- Yang FJ, Yao J, Min JJ, Li JH, Chen XQ (2016) Synthesis of high saturation magnetization FeCo nanoparticles bypolyol reduction method. Chem Phys Lett 648: 143-146.
- Karipoth P, Thirumurugan A, Joseyphus RJ (2013) Synthesis and magnetic properties of flower-like FeCo particles through a one pot polyol process. J Colloid Interface Sci 404: 49-55.
- Abbas M, Islam MN, Rao BP, Ogawa T, Takahashi M, et al. (2013) One-pot synthesis of high magnetization air-stable FeCo nanoparticles by modified polyol method. Mater Lett 91: 326-329.
- Ivanov YP, Vivas LG, Asenjo A, Chuvilin A, Chubykalo-Fesenko O, et al. (2013) Magnetic structure of a single-crystal hcp electrodeposited cobalt nanowire. Europhys Lett 102.
- Stahl B, Gajbhiye NS, Wilde G, Kramer D, Ellrich J, et al. (2002) Electronic and magnetic properties of monodispersed FePt nanoparticles. Adv Mater 14: 24-27.
- Kumar PA, Mitra S, Mandal K (2007) Structural and magnetic properties of Co nanoparticles in Cu/SiO2 matrix. Indian J Pure Appl Phys 45: 21-26.
- Kurlov AS, Gusev AI, Rempel AA (2012) Morphology of ultrafine cobalt and nickel powders. Rev Adv Mater Sci 32: 52-60.
- Ponrouch A, Bichat MP, Garbarino S, Maunders C, Botton G, et al. (2010) Synthesis and characterization of well aligned Ru nanowires and nanotubes. ECS Trans. 25: 3-11.
- onrouch A, Bichat MP, Garbarino S, Maunders C, Botton G, et al. (2010) Synthesis and characterization of well aligned Ru nanowires and nanotubes. ECS Trans. 25: 3-11.
- Baraton MI, Uvarova IV (2001) Functional gradient materials and surface layers prepared by fine particles technology, (2nd edtn), Springer, Ukraine.
- Kolhatkar AG, Jamison AC, Litvinov D, Willson RC, Lee TR (2013) Tuning the magnetic properties of nanoparticles. Int J Mol Sci 14: 15977-16009.
- Liu QF, Jiang CJ, Fan XL, Wang JB, Xue DS (2006) Low temperature magnetic properties of Co antidot array. Chin Phys Lett 23: 1592-1595.
- Cantillo CO, Miranda ANS, Perez OP, Xin Y (2012) Size- and phase-controlled synthesis of cobalt nanoparticles for potential biomedical applications. J Appl Phys 111: 07B324.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi