Research Article, J Nanomater Mol Nanotechnol Vol: 12 Issue: 1
Profenofos Loaded Nanoliposomes against Agricultural Insect Pest
Sneha N1, Ravikumar Hosamani1*, Chandrashekhar SS2 and Udikeri SS3
1Department of Biotechnology, University of Agriculture Sciences, Dharwad, India
2Department of Seed Science and Technology, University of Agriculture Sciences Dharwad, India
3Department of Agriculture Research Station, University of Agriculture Sciences Dharwad, Dharwad, India
*Corresponding Author: Ravikumar Hosamani, Department of Biotechnology
University of Agriculture Sciences, Dharwad, India
Tel: +91 836-2214441
E-mail: hosamanirr@uasd.in
Received date: 22 August, 2022, Manuscript No. JNMN-22-72631; Editor assigned date: 25 August, 2022, PreQC No. JNMN-22-72631 (PQ); Reviewed date: 08 September, 2022, QC No. JNMN-22-72631; Revised date: 10 January, 2023, Manuscript No. JNMN-22-72631 (R); Published date: 20 January, 2023, DOI: 10.4172/2324-8777.1000353
Citation: Sneha N, Hosamani R, Chandrashekhar SS, Udikeri SS (2023) Profenofos Loaded Nanoliposomes against Agricultural Insect Pest. J Nanomater Mol Nanotechnol 12:1.
Abstract
Nano encapsulation technology enables developing formulations that exhibit slow and persistent release for sustainable agricultural pest management. The aim here was to synthesize, characterize and evaluate a nano encapsulated profenofos formulation for its slow release efficacy. Technical grade profenofos at three concentrations were nanoencapsulated using soya lecithin and the resultant data revealed nanoscale and spherical shape liposome formation Further, in vitro stability studies showed significantly slower active ingredient’s release (76.99 ± 8.20% vs.100% on 11th day) and photo degradation rate (66.77% ± 3.4% vs. 100% on 13th day) in liposome encapsulated profenofos. Bio efficacy tests indicated liposome encapsulated formulation rendered persistent toxicity for a longer period on Spodoptera litura compared to control. In specific, the Median Survival (MS) rate in liposome encapsulated 0.174% profenofos was 50% more with 3.051 fold less toxic (hazard ratio) compared to control. Similarly, comparable results were observed in liposome encapsulated 0.084% and 0.043% profenofos. Thus, nanoencapsulated formulation can be used as a slow release pesticide avoiding repetitive applications.
Keywords
Nanotechnology; Pest management; Bio efficacy; Slow release; Spodoptera litura
Introduction
Synthetic pesticides have contributed significantly to enhancing crop protection and crop yield. However, post application, the rapid loss of active ingredients due to photolysis, hydrolysis, microbial degradation, evaporation, leaching and volatilization leads to reduced efficacy and accumulated health and environmental hazard [1-3]. The complete elimination of these effective pesticides from use is detrimental to food security. While we pursue biotechnological, biological control and Integrated Pest Management (IPM) strategies for sustainable pest management, alternatively refurbishing leading synthetic pesticide products into new formulations without compromising their efficacy is also a good economic strategy. Nano encapsulation technology enables developing such formulations, essentially it is a technique where insecticide or any other bioactive molecules entrapped within a carrier material can deliver to the specified target at a slower and persistent rate for a longer duration. Nano encapsulation offers several advantages over microencapsulation. In particular, a thin encapsulation layer should significantly improve mass transport of active ingredients to the target site and reduce the total volume of non active ingredient compounds that is transported. In addition, specifically with nano encapsulation of pesticides, the small size of the capsules will ensure easy penetration into alternative sites to bring about effective pesticide action [4,5]. The promising encapsulation materials for insecticide delivery are polymer, lipids and porous inorganic and clay based materials [6]. Although the use of liposome mediated insecticide delivery reports is limited, the biomedical research community has widely exploited slow release efficacy and better encapsulation properties for targeted drug delivery [7,8]. Liposomes are self assembled microscopic spherical shaped vesicles creating a central aqueous cavity surrounded by lipid membrane/s or lamella/s. Liposomes can be synthesized using natural and/or synthetic lipids that are biodegradable and biocompatible. These unique bilayer liposomes have enabled us to use them as a carrier for hydrophilic and lipophilic molecules. A lipophilic molecule entraps within the lipid bilayer/membrane whereas; hydrophilic molecules entrap inside the aqueous cavity of the lipid membrane. These liposomal formulations are widely recognized as superior drug carriers and extensively used in the medical and pharmaceutical industries mainly because of cost effectiveness, compatibility with cell macromolecules and ease of functionalization [9]. In some cases, liposome encapsulated drugs are already in preclinical and clinical trials [10,11]. Despite its widespread usage in the medical industry, nano encapsulation technology is in limited use in agriculture applications, especially, for pesticide encapsulation. To date we found one published report patented liposome encapsulated pesticide formulation for agriculture pest management [12]. An unpublished report indicated an improved bio efficacy of liposome encapsulated bifenthrin against western corn rootworm [13]. Except for these two studies, there is no literature on the development of liposome encapsulated insecticide formulations and evaluation of their bio efficacy against agriculture insect pests. In the present study, we chose to test slow release bio efficacy of liposome formulation on 3rd instar Spodoptera litura (Fabrics) (Lepidoptera: Noctuid) larvae. Spodoptera litura being one of the major leaf feeding polyphagia’s pests with ease of rearing in the laboratory condition makes an ideal target pest for bioassay [14-16]. We hope these in house synthesized, characterized and evaluated slow release profenofos loaded nano liposomes against 3rd instar Spodoptera litura larvae in laboratory conditions will help in future evaluation in greenhouse and field conditions for sustainable agricultural pest management application in the future.
Materials and Methods
Synthesis of liposome encapsulated profenofos was carried out using the thin film hydration method [17]. The materials used for the synthesis were soy phospholipids, cholesterol extra pure (98% pure, MW 386.66), solvents dichloromethane (MW 84.93), acetone obtained from Sisco research laboratories Pvt. Ltd. (New Mumbai, India); technical grade Profenofos of (94% purity, C11H15BrClO3PS, MW 373.63) obtained from Meghmani Organics Limited (Ahmadabad, India). For characterization, Particle Size Analyzer (PSA), Scanning Electron Microscopy (SEM) and UV-Visible spectrophotometer used from the green nanotechnology laboratory university of agricultural sciences, Dharwad, India.
Synthesis and characterization of liposome encapsulated profenofos
Synthesis of liposomes carried out using a thin film hydration method called Bangham method. A mixture of soy phospholipids and cholesterol in various ratios (by weight) and different concentrations of profenofos dispersed in a 50 ml beaker containing 10 ml dichloromethane by agitating for 15 minutes. Then the organic solvent was removed under vacuum with a rotary evaporator for 30 minutes at 200 Revolutions Per Minute (RPM) at 40°C in a water bath. The resultant dried lipid film was kept open overnight to remove the leftover traces of dichloromethane. Then the dried lipid film was rehydrated with 20 ml of DI water under vigorous shaking for 1 hour at 350 rpm at 45°C to obtain multilamellar liposomes. Then, the liposomal samples were subjected to probe sonication at 15 amps for 30 minutes and at 50°C to yield unilamellar liposomes. Similarly, lipid film with no insecticide rehydrated with water only to obtain blank liposomes for comparison. Various concentrations of soy phospholipids, cholesterol and profenofos and/or in combinations used to achieve better encapsulation efficiency and particle size less than 100 nm.
Experimental detail
For profenofos loaded nanoliposome synthesis, different ratios of soy phospholipids and cholesterol used were 2:1, 3:2, 4:3, 5:4 and 6:5. Based on the optimum nanoliposome formation, the 5:4 ratio was further used to entrap profenofos. Since the recommended concentration of profenofos in the field condition is 0.20% per liter, three different concentrations with not exceeding recommended dose was used i.e., 0.174, 0.084, 0.043% (corrected to percent encapsulation). All the synthesis experiments were carried out at least three times to ensure statistical reproducibility. Detailed characterization carried out using various analytical and imaging techniques. Morphology of liposome encapsulated profenofos was observed using a light microscope. Briefly, after synthesis, a small quantity (5 µl) of the sample was placed on a glass slide with a coverslip on it and observed for their shape at 10X under a light microscope. A particle size analyzer (Nicomp NANOZ Z3000 PSS) was used to determine the average size distribution of synthesized liposomes. For Scanning Electron Microscope (SEM) images, liposome encapsulated profenofos samples were placed on the aluminum foil and allowed to dry at room temperature. Dried samples were then coated on metal stabs with carbon tape and sputter coated with gold. Further, the samples were subjected to SEM imaging under vacuum and examined for surface morphology (Carl Zeiss-EVO-18- UK). The optical absorption of synthesized liposomes was measured using a UV-Visible spectrophotometer in the range of 200 nm-750 nm wavelengths at room temperature.
Determination of Encapsulation Efficiency (EE) and insecticide content in the liposomes
Liposome encapsulated profenofos samples were ultra centrifuged at 1500 rpm for 30 minutes to separate free drugs. The supernatant containing free drug discarded and aggregated liposomes settled at the bottom collected for further processing. Then, the aggregated samples dissolved in methanol to disrupt the lipid bilayer and released profenofos active ingredient quantified using UV-spectrophotometer. Parallelly, we used insecticide samples (0.2%, 0.1% and 0.05%) as a standard for calibration. Based on the absorbance values of both profenofos per se and liposome-encapsulated profenofos, encapsulation efficiency was calculated using the following formula [18].

In vitro stability studies: Release rate and photo degradation rate
The barrier diffusion method was used for determining the active ingredient's release rate from liposomes in this study [19]. Briefly, 40 mg of dried liposome encapsulated profenofos was weighed and placed in a dialysis bag filled with a 2 ml release medium (Phosphate buffer, pH 7.0+20% methanol). Then, both the ends of the dialysis tube were sealed and placed in a conical flask containing a 150 ml release medium. The whole set up kept under constant shaking at 120 rpm at 25°C in the dark. At 0, 1, 3, 5, 7, 9, 11, 13, 15 and 17 days intervals, 2 ml release medium pipetted out from the conical flask and determined the concentration of the released insecticide rate. Similarly, the photodegradation rate of the sample was also determined on alternate days (at 0, 1, 3, 5, 7, 9, 11, 13, 15, 17, 19, 21 days) and these experiments lasted for 21 days. For assessing photodegradation rate, different concentrations of liposome encapsulated profenofos, profenofos per se mixed with methanol, placed in a small glass beaker and kept open under natural light. The photodegradation rate was determined using a spectrophotometer on alternate days from 0 days to until 21st day. Based on the OD values, the degradation rate was determined by comparing liposome encapsulated profenofos with profenofos per se.
Bio efficacy study: Spodoptera litura
The different instar Spodoptera litura larvae were meticulously cultured on castor leaves in well maintained insectary (laboratory conditions) at ARS, Dharwad farm using standard insect rearing method [20]. The average temperature of the laboratory during rearing and bioassays was 28°C ± 3°C and 65%-70% relative humidity. Freshly collected castor leaves from the field were washed thoroughly with water and air dried and then larvae were allowed to feed on them. Specifically, 3rd instar larvae of the F2 generation were used for all bio efficacy studies. For bio efficacy studies, we employed a leaf dip method, briefly, about 1 cm diameter castor leaf discs were prepared from air dried leaves and discs were gently dipped in different test solutions for 90 seconds and air dried on the tissue paper for 30 minutes [21]. These treated leaf discs were later placed in the petri plates of about 90 x 15 mm size and twenty 3rd instar synchronized larvae per petri plate in triplicate were released and allowed to feed on them. At every 12 hours, mortality was recorded in both conditions (test and control conditions) [22]. Laboratory based bioassay includes the following nine test groups: Three different concentrations of profenofos 0.174, 0.084, 0.043% per se, three liposome encapsulated 0.174%, 0.084%, 0.043% profenofos and one each liposomes encapsulated water, water per se and acetone (25%) per sec. Three independent biological replicates with a total of 60 larvae were used for statistical analysis. Finally, all the bioassay data were subjected to survival analysis to determine the efficacy using graph pad prism program version 8.2.1.
Results
Synthesis and characterization of liposome encapsulated profenofos
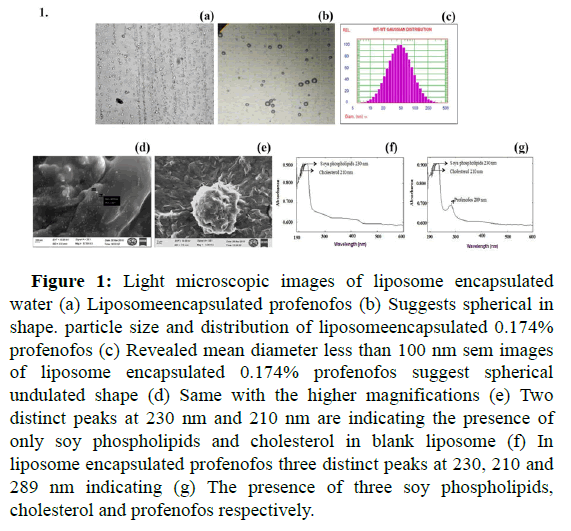
A mixture of soy phospholipids+cholesterol with 5:4 and profenofos (0.2%, 0.1% and 0.05%) yielded multilamellar liposomes. Multilamellar liposomes subjected to probe sonication further yielded a significant amount of unilamellar liposomes. Morphology of liposome encapsulated profenofos and liposome encapsulated water (blank liposomes) under a light microscope (10X) revealed liposomal particles were spherical and varied in size. Based on the image, Profenofos was entrapped within the lipid bilayer of the liposomes because of its hydrophobic nature (Figure 1 and Tables 1 and 2).
| Mean particle size (nm) of liposomes with concentrations of profenofos | |||
|---|---|---|---|
| Soy phospholipids+cholesterol | 0.20% | 0.10% | 0.05% |
| 2:01 | 369.80 ± 4.56 | 553.10 ± 3.45 | 480.00 ± 4.97 |
| 3:02 | 183.20 ± 1.98 | 250.10 ± 4.21 | 269.30 ± 2.54 |
| 4:03 | 205.10 ± 2.06 | 165.70 ± 1.99 | 230.00 ± 3.01 |
| 5:04 | 56.90 ± 4.86 | 61.90 ± 3.48 | 79.20 ± 5.82 |
| 6:05 | 148.00 ± 1.27 | 129.00 ± 1.08 | 135.00 ± 2.31 |
| *EE (%) for 5:4 ratio | 87.02 ± 2.45 | 84.32 ± 1.28 | 86.97 ± 2.34 |
| Corrected profenofos concentration | 0.17% | 0.08% | 0.04% |
Note: (* EE (%) – Encapsulation efficiency and corrected profenofos concentration)
Table 1: Mean particle size (nm) of three (0.2%, 0.1% and 0.05%) tested concentrations of liposome encapsulated profenofos obtained using five different ratios (2:1, 3:2, 4:3, 5:4 and 6:5) of soy phospholipids and cholesterol combination.
| Time dependent particle size analysis (nm) of varied concentrations of liposome encapsulated profenofos | |||
|---|---|---|---|
| Days | 0.17% | 0.08% | 0.04% |
| 1 | 44.80 ± 3.62 | 65.40 ± 4.83 | 79.50 ± 4.52 |
| 5 | 57.60 ± 2.17 | 66.00 ± 2.16 | 80.70 ± 1.08 |
| 10 | 61.30 ± 4.12 | 67.20 ± 3.45 | 85.20 ± 2.41 |
| 15 | 67.70 ± 3.54 | 72.50 ± 2.84 | 90.40 ± 4.28 |
| 20 | 79.90 ± 1.28 | 82.80 ± 1.58 | 93.10 ± 2.43 |
Table 2: Time dependent particle size analysis revealed liposome encapsulated profenofos at three different concentrations (0.174%, 0.084% and 0.043%) remained stable with less than 100 nm Nano scale up to 20 days at room temperature.
Figure 1: Light microscopic images of liposome encapsulated water (a) Liposomeencapsulated profenofos (b) Suggests spherical in shape. particle size and distribution of liposomeencapsulated 0.174% profenofos (c) Revealed mean diameter less than 100 nm sem images of liposome encapsulated 0.174% profenofos suggest spherical undulated shape (d) Same with the higher magnifications (e) Two distinct peaks at 230 nm and 210 nm are indicating the presence of only soy phospholipids and cholesterol in blank liposome (f) In liposome encapsulated profenofos three distinct peaks at 230, 210 and 289 nm indicating (g) The presence of three soy phospholipids, cholesterol and profenofos respectively.
Encapsulation Efficiency (EE) and insecticide content in the liposomes
In the case of blank liposomes, we found 2 peaks at 230 nm and 210 nm indicating the presence of only soy phospholipids and cholesterol. Whereas, in the liposomeencapsulated profenofos sample, we found three distinct peaks at 230 nm, 210 nm and 289 nm indicating the presence of three compounds i.e., soy phospholipids, cholesterol and profenofos respectively. However, after confirming profenofos entrapment within the liposomes, Encapsulation Efficiency (EE) was calculated. The results indicated that EE increased with an increased ratio of soy phospholipids and cholesterol. Among the various ratios of phospholipids and cholesterol used (2:1, 3:2, 4:3, 5:4 and 6:5) the EE was highest at 5:4 ratios. In liposome encapsulated 0.20%, profenofos, EE was 87.02 ± 2.45%. In the case of 0.1% and 0.05% profenofos loaded liposomes, EE was highly comparable i.e., 84.32 ± 1.28% and 86.97 ± 2.34% respectively. Based on the EE, the concentration of profenofos entrapped within liposomes was corrected and corrected concentrations of profenofos were -0.174% (instead of 0.2%), 0.084% (instead of 0.1%) and 0.043% (instead of 0.05%).
The release rate and photodegradation of liposome encapsulated profenofos
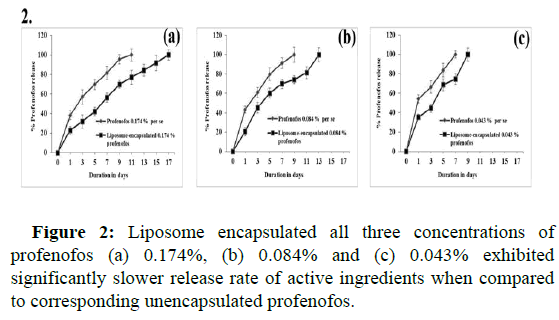
The release rate of active ingredients studies revealed a 100% release rate of profenofos on the 11th day in the 0.174% profenofos per se treatment group. Whereas, in liposome encapsulated (0.174% profenofos), it was 76.99% ± 8.2% on the same day (11th day) (p=0.026) and a 100% release rate was observed on the 17th day. In 0.084% profenofos per se, 100% active ingredients release rate was observed on the 9th day, while liposome-encapsulated (0.084% profenofos) group extended up to 13th day (p=0.0046). Similarly, in 0.043% profenofos per se, a 100% release rate was observed on the 7th day and in the liposome encapsulated (0.043% profenofos) group, a 100 % release rate was observed on the 9th day (p=0.031), collectively, this data suggests that liposome encapsulated all three concentrations of profenofos exhibited a significantly slower release rate of active ingredients when compared to unencapsulated profenofos (Figure 2).
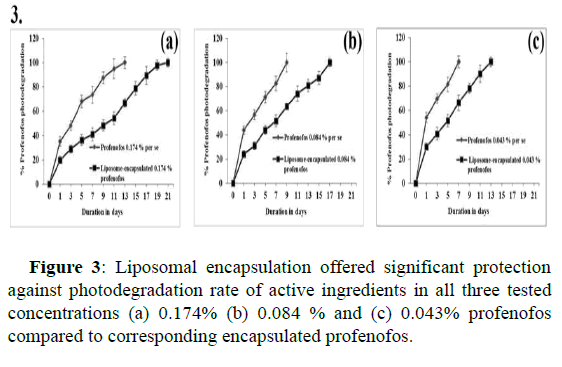
Photo degradation data revealed that 100% active ingredients of 0.174% profenofos alone were completely degraded within 13 days, while the liposome encapsulated with the same conc. of profenofos showed a slower degradation rate (66.77% ± 3.4%) at the same test duration (p-value 0.006). Interestingly, 100% degradation of the same concentration extended until the 21st day. Similarly, in the case of 0.084% and 0.043% profenofos per se, 100% degradation of profenofos was observed at the 9th and 7th days respectively. While the same concentration of liposome encapsulated profenofos (0.084% and 0.043%) showed a degradation rate of 63.88% ± 2.4% and 66.31% ± 6.1% respectively at the same time intervals (9th and 7th day). Further, a 100% degradation rate was observed on the 17th and 13th day of the experiment (p-value of 0.0082 and 0.0027) respectively. Thus, liposomal encapsulation offered significant protection against photodegradation of all the tested profenofos concentrations (Figure 3).
Slow release efficacy of liposome encapsulated profenofos against Spodoptera litura larvae
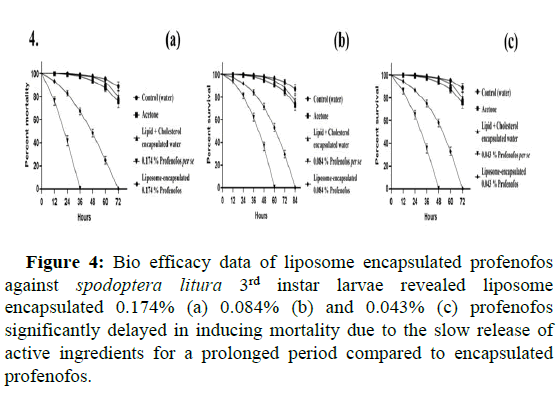
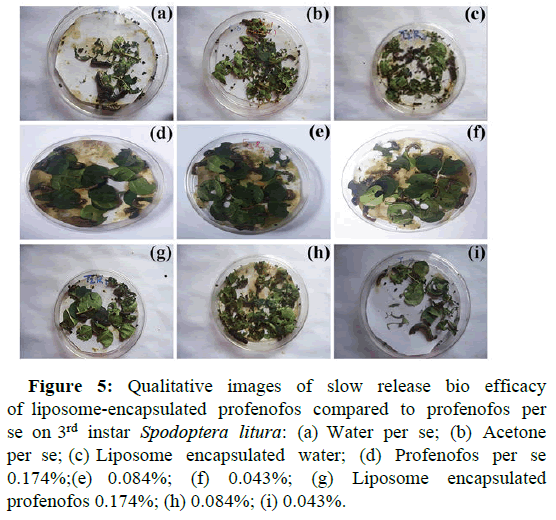
Bio efficacy data of liposome encapsulated profenofos indicated a significant delay in inducing mortality in Spodoptera litura larvae due to the slow release of active ingredients for a prolonged period compared to unencapsulated profenofos. For bio efficacy studies, profenofos per se (unencapsulated) compared with varied concentrations of liposome encapsulated profenofos for median survival rate and Hazard Ratio (HR). Results indicated that, median survival time for 0.174% profenofos per se was 24 h, whereas it was 48 h for liposome encapsulated (0.174%) profenofos with a median survival ratio of 0.50% and 95% Confidence Interval (CI) ratio ranged between 0.398-0.627. The hazard ratio between profenofos 0.174% per se and liposome encapsulated (0.174%) profenofos, was 3.051:1, indicating 3 fold higher toxicity of profenofos per se with 95% CI of 2.264-4.111 (p=0.000129), (Table 3). Similarly, the median survival time for 0.084% profenofos per se was 36 h, while it was 60 h for liposome encapsulated (0.084%) profenofos with a median survival ratio of 0.60 and the 95% CI ratio between 0.480-0.748. Hazard ratio between 0.084% profenofos per se and liposome encapsulated (0.084%) profenofos was 2.655:1 with 95% CI of 2.051-3.437 (p=0.00336). Further, the results for 0.043% profenofos per se and liposome encapsulated (0.043%) profenofos showed the same median survival rate as that of 0.084% data with the 95% CI ratio for the median survival was 0.467-0.770. The hazard ratio was 2.562 with 95% CI of HR showing 1.905-3.4446 (p=0.00401), (Table 4 and Figure 4). The qualitative bioassay images of all the test concentrations are depicted in detail. In totality, liposome encapsulated profenofos offered prolonged toxicity to Spodoptera litura larvae (Figure 5).
| Groups | MS (hrs) | MS ratio | 95% CI of ratio | Hazard Ratio (HR) | 95 % CI of HR | Log rank test p-value |
|---|---|---|---|---|---|---|
| 0.174 % Profenofos per se | 24 | 0.5 | 0.40- 0.63 | 3.05 | 2.26-4.11 | 0.000129 *** |
| Liposome encapsulated 0.174% profenofos | 48 | |||||
| 0.084% Profenofos per se | 36 | 0.6 | 0.48-0.75 | 2.66 | 2.05-3.44 | 0.00336 *** |
| Liposome-encapsulated 0.084% profenofos | 60 | |||||
| 0.043 % Profenofos per se | 36 | 0.6 | 0.47-0.77 | 2.56 | 1.91-3.44 | 0.00401 *** |
| Liposome encapsulated 0.043% profenofos | 60 |
Note: (Here indicated highly significant differences among profenofos per se and liposome encapsulated profenofos in terms of mean survival rate and hazard ratio as quantified using graph pad prism survival analysis program (n=15 and in triplicates; Kaplan-Meier survival plot with the log rank test: P<0.05)
Table 3: Median survivals (MS) and Hazard Ratio (HR) of liposome encapsulated 0.174%, 0.084% and 0.043% profenofos compared to profenofos per se revealed lower and prolonged toxicity against Spodoptera litura 3rd instar larvae.
| Absorbance with at different wavelength range | |||||
|---|---|---|---|---|---|
| Sample | 235 nm | 240 nm | 245 nm | 250 nm | 255 nm |
| Dichloromethane | 0.430 ± 1.03 | 0.150 ± 2.15 | 0.071 ± 1.50 | 0.020 ± 1.42 | 0.010 ± 2.03 |
| 0.2% Liposome encapsulated profenofos | 0.005 ± 2.30 | 0.010 ± 1.20 | 0.004 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 |
| 0.1% Liposome encapsulated profenofos | 0.007 ± 1.61 | 0.003 ± 3.11 | 0.001 ± 1.11 | 0.000 ± 0.00 | 0.000 ± 0.00 |
| 0.05% Liposome encapsulated profenofos | 0.004 ± 2.84 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 |
Table 4: Residual analysis of dichloromethane in varied concentrations of liposome encapsulated profenofos (0.2, 0.1 and 0.05%).
Figure 4: Bio efficacy data of liposome encapsulated profenofos against spodoptera litura 3rd instar larvae revealed liposome encapsulated 0.174% (a) 0.084% (b) and 0.043% (c) profenofos significantly delayed in inducing mortality due to the slow release of active ingredients for a prolonged period compared to encapsulated profenofos.
Figure 5: Qualitative images of slow release bio efficacy of liposome-encapsulated profenofos compared to profenofos per se on 3rd instar Spodoptera litura: (a) Water per se; (b) Acetone per se; (c) Liposome encapsulated water; (d) Profenofos per se 0.174%;(e) 0.084%; (f) 0.043%; (g) Liposome encapsulated profenofos 0.174%; (h) 0.084%; (i) 0.043%.
Comparative analysis in terms of median survival rate and hazard ratio only among the liposome encapsulated concentrations (0.174%, 0.084% and 0.043%) was calculated. When we compared liposome encapsulated 0.174% and 0.084% profenofos, we found that mean survival time was 48 h and 60 h respectively with a ratio of 0.8 and a 95% CI between 0.653-0.980. The hazard ratio between 0.174% and 0.084% was 1.186:1.00 with a 95% CI of 0.969-1.452, it was marginally significant (p=0.0292). Similarly, a comparison between liposome encapsulated 0.174% and 0.043% profenofos showed a mean survival time of 48 and 60 h. Respectively with a ratio of 0.80 and 95% CI of 0.643-0.990. The hazard ratio between 0.174% and 0.043% liposome encapsulated profenofos was found to be 1.417:1.00 with a 95% CI of 1.148-1.748 (p=0.00112). However, in the comparison between liposome encapsulated 0.084% and 0.043% of profenofos, the mean survival time remained unchanged at 60 h with a ratio of 1:1 and 95% CI of 0.801-1.248. The hazard ratio is 1.914:1.00 with a 95% CI of 0.958-1.489 (p=0.0395). As anticipated, profenofos per se at varied concentrations (0.174, 0.084 and 0.043%) showed a significant change in mean survival rate when compared to acetone (25%) used for diluting profenofos. In specific, 0.174% profenofos showed mean survival rate of Spodoptera litura larvae of 76.29%, 41.3% and 0.00% at 12, 24 and 36 h respectively (p=0.000198). Similarly, at 0.084% profenofos, mean survival rate was observed to be 86.12%, 65.05%, 35.42% and 0.00% at 12, 24, 36 and 48 h (p=0.000114) respectively. At 0.043% profenofos, mean survival rate of 92.22%, 75.82%, 48.86%, 8.68% and 0.00% at 12, 24, 36, 48 and 60 h respectively (p=0.00101) was observed. The mean survival rate of Spodoptera litura larvae exposed to varying concentrations of liposome encapsulated profenofos when compared with liposome encapsulated water (blank liposomes) showed no significant change in all the tested concentrations and exposure time. However, liposome encapsulated 0.174% (p=0.00034), 0.084% (p=0.00028) and 0.043% (p=0.0019) profenofos showed significantly lower mean survival rate compared to blank liposomes.
Discussion
The present study reports synthesis and characterization of liposome encapsulated profenofos and their bio efficacy against 3rd instar Spodoptera litura larvae in laboratory conditions. In previous studies, various natural polymeric materials such as chitosan, alginates, starch, polylactic acid and lipids were used as wall material to encapsulate medically important drugs, bioactive compounds and conventional insecticides to achieve controlled release of active ingredients [23-27]. Many of these studies used the thin film hydration method for synthesizing liposomes and found it to be the simplest and efficient method for encapsulation of various bioactive compounds [28,29]. Similarly, in the present study, the thin film hydration method was followed with minor modifications for the synthesis of liposome encapsulated profenofos. Profenofos (C11H15BrClO3PS, IUPAC name O-(4-Bromo-2-chlorophenyl) O-ethyl S-propyl phosphorothioate) is an organophosphate insecticide [30]. Also called Phosphorothioic acid, O-(4-Bromo-2-chlorophenyl) O-ethylS-propyl ester, is being used on various crops such as cotton, potato, maize, soya bean, sugar beet, etc., against lepidopteron pests [31]. Based on the literature, we chose to test various ratios of soy phospholipids and cholesterol for liposome synthesis. While soy phospholipids are the main constituents of the liposomal bilayer membrane that influence the size of the liposomes, cholesterol combination with it gives better rigidity to the bilayer and helps for longer retention of the entrapped compound. Among the tested ratios, the 5:4 ratio of soy phospholipids and cholesterol yielded the highest encapsulation efficiency and particle size distribution on the nanoscale. This encapsulation and size distribution data are in accordance with the earlier study carried out by. The lower ratios of phospholipids and cholesterol yielded lower percent encapsulation with increased particle size distribution. This suggests that the optimum ratio of soy phospholipids and cholesterol and interaction with the entrapping compound is critical in determining the size of liposomal particles. Since, different ratios of soy phospholipids and cholesterol was used, various mean diameters of liposomal particles and different concentrations of profenofos entrapment was obtained. However, the shape of the liposome remained spherical and this shape is justified because soy phospholipids have an inherent ability to form a spherical shape when it comes in contact with an aqueous solution. These spherical vesicles were different in size and multiple layered called multilamellar vesicles as visualized in an optical microscope. These observations are in accordance with the unpublished report. Mean diameter of >80% distribution of liposomes clearly showed less than 100 nm in size indicating efficient liposome synthesis protocol. Further, SEM images of liposome particles revealed a spherical shape. However, in the magnified version of SEM images, we found ununiformed lipid encapsulation/coating. Part of the reason for this could be dehydration of the hydrophilic substance within the liposomes after drying the liposomal samples. The uniformity of lipid encapsulation also depends on the interaction of entrapped compounds and lipids. Thus, a better understanding of the chemistry between these two compounds may enhance the uniform coating of lipid molecules on the test compound. Typically, soy phospholipids and cholesterol will absorb at 230 and 210 nm, whereas profenofos absorbs at 289 nm [32]. Similarly in our study, we found multiple peaks at 230, 210 and 289 nm in liposome encapsulated profenofos sample suggesting the presence of all three i.e., soy phospholipids, cholesterol and profenofos respectively. However, in the case of blank liposomes, we found only two peaks at 230 and 210 nm indicating the presence of soy phospholipids and cholesterol only. One of the main objectives for encapsulation of profenofos is to achieve a slower release rate of active ingredients and reduced photodegradation. In the present study, we found a significant delay in the release rate and reduced photodegradation in liposome encapsulated profenofos when compared to naked profenofos. It indicates that considerable protection is offered by liposomal bilayer encapsulation against photodegradation and facilitated the slow release of active ingredients. Similarly, in vitro stability of liposome encapsulated water soluble compounds of cordyceps sinensis revealed a significantly slower release rate compared to its free form. Microencapsulated cypermethrin showed slower release rate in lower core to shell ratio i.e., 2.5:1 [33]. The slow release rate of active ingredients and delayed photodegradation rate were also reported in other studies where other lipids were used as wall material. For instance, microencapsulated chlorpyriphos using sodium alginate and starch significantly delayed the release rate of active ingredients in comparison with free chlorpyriphos. In another study, encapsulated dichlorvos and chlorpyrifos using starch based silver nanoparticles for enhanced insecticide delivery was reported [34]. Imidachloprid encapsulation with alginate and chitosan showed eight times longer release rate when compared with Imidachloprid alone [35]. Azadiractin encapsulated with alginate spheres with starch and polyethylene glycol showed a slower release rate when compared to uncoated Imidachloprid [36]. Similarly, reduced photodegradation of active ingredients was achieved using wall materials such as polylactic acid encapsulated spinosad and emamectin benzoate, alginate coated Imidachloprid, nanocapsules loaded with natural abamectin and pyrethrin insecticide [37,38]. Liposome encapsulated profenofos offered prolonged toxicity. Bio efficacy data of liposome encapsulated profenofos indicated a significant delay in inducing mortality in Spodoptera litura larvae due to the slow release of active ingredients from liposomal formulation and retained toxicity for a longer duration compared to unencapsulated profenofos. This directly correlates with the slower release rate and reduced photodegradation rate observed in liposome encapsulated profenofos from this study. Thus, liposome encapsulated profenofos offered significantly improved long term toxicity to Spodoptera litura larvae. The microencapsulated bifenthrin within the liposomes showed a decrease in the mortality rate of western corn rootworm due to the slow release of bifenthrin into the soil (13, unpublished report). Such bio efficacy studies were also carried out using different encapsulating wall materials such as chitosan, polylactic acid, alginate, etc. against agriculture insect pests. For instance, reported that insecticidal activity of imidachloprid (50%) encapsulated with chitosan and alginate was more effective in terms of toxicity when compared with technical grade imidachloprid (95%) against martianus dermestoide [39]. Reported that nanocapsules loaded with garlic essential oil were found to be effective after five months. More recently, it was reported that microencapsulated spinosad and emamectin benzoate with polylactic acid showed long- term toxicity against Plutella xylostella up to 17 days, while the toxicity of encapsulated spinosad and emamectin benzoate lasted only for 9 days. Reported residue and bio efficacy studies of controlled release formulations of imidacloprid against soybean pests. Many of these studies although used different wall materials for encapsulation, it correlates well with slow toxicity data offered by liposome encapsulated profenofos in the present study. Thus, the current study's bio efficacy data is promising in sustainable agriculture pest management through the action of the slow release formulation. However, greenhouse and field conditions studies are warranted for further validation of its slow release bio efficacy. The development of such slow release encapsulated insecticide formulation also helps in lessening the chances of insect pest resistance to the insecticide. Such formulations are likely to be quite ideal in arid and semi arid tropics where many field crops are grown under high temperature regimes and have significant resistance issues. Liposomal Nano encapsulation offers an added advantage of rain fastness and stickiness that are essential in high rainfall and temperate zones also. Although insecticide resistance monitoring is done quite frequently for Spodoptera litura and other insect pests, it is difficult to manage insecticide resistance sustainably because of the lack of field level synergists, warranting new chemistry pesticides. Alternatively, combination products of two insecticides gain importance. In either case, insecticide resistance issues remain unsolved, thus, slow release insecticide formulation inspired by nanotechnology may promise to be better Insecticide Resistance Management (IRM) strategies in the future.
Conclusion
Among the various tested ratios, the 5:4 ratio of soy phospholipids and cholesterol showed particle size in nanoscale i.e., below 100 nm with the highest encapsulation efficiency of >80 percent. The spherical shape of the synthesized liposome encapsulated profenofos was evident from morphological characterization data of light microscopy and Scanning Electron Microscopy (SEM). UV-Vis absorption spectra of profenofos loaded liposomes revealed three peaks at 230 nm, 210 nm and 289 nm confirming the presence of soy phospholipids, cholesterol and profenofos respectively. When compared to profenofos alone, profenofos loaded liposomes offered significant control on the release rate of active ingredients and reduction in the photo degradation rate under laboratory conditions. Bio efficacy data revealed, profenofos loaded liposome at all the tested concentrations significantly prolonged toxicity as evident from the reduced mortality rate of spodoptera litura larvae due to the slow release of profenofos active ingredient when compared with profenofos alone.
Acknowledgment
Authors acknowledge green nanotechnology lab for generously sharing their facility to carry out this research and second author’s Master’s research advisory committee for their suggestions through the research program.
Declaration of Interest
The authors declare that there is no conflict of interest.
Author’s Contribution
RH and CSS conceived and designed the research. SN conducted the experiments. RH and SN analyzed the data and drafted the manuscript. RH, CSS and USS supervised the experiments and critically read the manuscript. All authors read and approved the manuscript.
References
- Moghul MG, Akin H, Hasirci N, Trantolo DJ, Gresser JD, et al. (1996) Controlled release of biologically active agents for purposes of agricultural crop management. Resour Conserv Rec 16:289-320.
- Scher HB (1999) Controlled-release delivery systems for pesticides. 1st Edition, CRC Press. New York, 366.
- Campos EVR, De Oliveira JL, Fraceto LF (2014) Applications of controlled release systems for fungicides, herbicides, acaricides, nutrients and plant growth hormones: A review. Adv Sci Eng Med 6:373-387.
- Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, et al. (2010) Nanoparticulate material delivery to plants. Plant Sci 179:154-163.
- Gogos A, Knauer K, Bucheli TD (2012) Nanomaterials in plant protection and fertilization: Current state, foreseen applications and research priorities. J Agric Food Chem 60:9781-9792.
[Crossref] [Google Scholar] [PubMed]
- Ghormade V, Deshpande MV, Paknikar KM (2011) Perspectives for nano biotechnology enabled protection and nutrition of plants. Biotechnol Adv 29:792-803.
[Crossref] [Google Scholar] [PubMed]
- Fathi M, Mozafari MR, Mohebbi M (2012) Nanoencapsulation of food ingredients using lipid-based delivery systems. Trends Food Sci Technol 23:13-27.
- Tamjidi F, Shahedi M, Varshosaz J, Nasirpour A (2013) Nanostructured Lipid Carriers (NLC): A potential delivery system for bioactive food molecules. Innov Food Sci Emerging Technol 19:29-43.
- Banerjee R (2001) Liposomes: Applications in Medicine. J Biomater Appl 16:3-21.
- Samad A, Sultana Y, Aqil M (2007) Liposomal drug delivery systems: An updated review. Curr Drug Deliv 4:297-305.
[Crossref] [Google Scholar] [PubMed]
- Maurer N, Fenske DB, Cullis PR (2001) Developments in liposomal drug delivery systems. Expert Opin Biol Ther 1:923-947.
[Crossref] [Google Scholar] [PubMed]
- Spencer JL, Fouly H, Ahmad I, Liu GL (2016) Reducing insecticide resistance: Development of unique Liposomes Pest Control SYSTEM (LPCS). Dudley Smith Initiative.
- Ahmad M, Ghaffar A, Rafiq M (2013) Host plants of leaf worm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) in Pakistan. Asian J Agric Biol 1:23-28.
- Baskar K, Muthu C, Raj GA, Kingsley S, Ignacimuthu S, et al. (2012) Ovicidal activity of Atalantia monophylla (L.) Correa against Spodoptera litura Fab. (Lepidoptera: Noctuidae). Asian Pac J Trop Bio 2:987-991.
[Crossref] [Google Scholar] [PubMed]
- Daniel AJ, Samiayyan K (2017) Growth parameter indices of cutworm larva Spodoptera litura (Fab.) on various host plants. Int J Agric Sci 9:4372-4376.
- Bangham A, Standish MM, Watkins J (1965) Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol 13:238-252.
[Crossref] [Google Scholar] [PubMed]
- Huang BB, Zhang SF, Chen PH, Wu G (2017) Release and degradation of microencapsulated spinosad and emamectin benzoate. Sci Rep 7:10864.
[Crossref] [Google Scholar] [PubMed]
- Janusz W, Patrick P (2000) A novel in vitro release technique for the peptide containing biodegradable microspheres. AAPS Pharm Sci Tech 1:30-40.
[Crossref] [Google Scholar] [PubMed]
- Garad GP, Shivpuje PR, Bilapate GG (1984) Life fecundity tables of Spodoptera litura (Fabricius) on different hosts. Proc Ani Sci 93:29-33.
- Escoubas P, Lajide L, Mitzutani J (1993) An improved leaf‐disk antifeedant bioassay an its application for the screening of Hokkaido plants. Entomol Exp Appl 1993:99-107.
- Tukaram AH, Hosamani AC, Naveena R, Santoshagowda GB (2014) Bioassay of flubendiamide on Spodoptera litura (Fabricius) population collected from different host crops. Int J Environ Sci Technol 3:2225-2230.
- Neri-Badang MC, Chakraborty S (2019) Carbohydrate polymers as controlled release devices for pesticides. J Carbohydr Chem 38:67-85.
- Nuruzzaman M, Rahman MM, Liu Y, Naidu R (2016) Nanoencapsulation, nano guard for pesticide: A new window for safe application. J Agric Food Chem 64:1447-1483.
[Crossref] [Google Scholar] [PubMed]
- Roy A, Bajpai J, Bajpai AK (2009) Dynamics of controlled release of chlorpyrifos from swelling and eroding biopolymeric microspheres of calcium alginate and starch. Carbohydr Polym 76:222-231.
- Panwar P, Pandey B, Lakhera PC, Singh KP (2010) Preparation, characterization and in vitro release study of albendazole-encapsulated nanosize liposomes. Int J Nanomed 5:101-108.
[Crossref] [Google Scholar] [PubMed]
- Gibis M, Ruedt C, Weiss J (2016) In-vitro release of grape seed polyphenols encapsulated from uncoated and chitosan coated liposomes. Food Res Int 88:105-113.
[Crossref] [Google Scholar] [PubMed]
- Wagner AA, Vorauer-Uhl K (2011) Liposome technology for industrial purposes. J Drug Deliv 5:591325-591334.
[Crossref] [Google Scholar] [PubMed]
- Shashidhar GM, Manohar B (2018) Nanocharacterization of liposomes for the encapsulation of water soluble compounds from Cordyceps sinensis CS1197 by a supercritical gas anti solvent technique. RSC Adv 8:34634-34649.
[Crossref] [Google Scholar] [PubMed]
- Jangle RD, Thorat BN (2013) Reversed phase high performance liquid chromatography method for analysis of curcuminoids and curcuminoid loaded liposome formulations. Indian J Pharm Sci 75:60-65.
[Crossref] [Google Scholar] [PubMed]
- Ahmed KS, Mikhail WZA, Sobhy HM, Radwan EMM, Salaheldin TA (2019) Impact of nanosilver profenofos on cotton leaf worm, Spodoptera littoralis(Boisd.) larvae. Bull Natl Res Cent 43:46.
- Nguyen TA, Tang DQ, Tin Doan DC, Dang MC (2016) Micro and nano liposome vesicles containing curcumin for a drug delivery system. Nat Sci Nanosci Nanotechnol 7:35003.
- Kamble V, Sawant M, Mahanwar P (2018) Microencapsulation of cypermethrin via interfacial polymerization for controlled release application. Mater Today: Proc 5:22621-22629.
[Crossref]
- Nnemeka I, Sha'Ato R, Tor-Anyiin TA, Nnamonu L, Beukes P, et al. (2015) Facile formulation of starch silver nanoparticle encapsulated dichlorvos and chlorpyrifos for enhanced insecticide delivery. New J Chem 40:1777-1784.
- Guan H, Chi D, Yu J, Li X (2008) A novel photodegradable insecticide: Preparation, characterization and properties evaluation of nano imidacloprid. Pestic Biochem Physiol 92:83-91.
- Jerobin J, Sureshkumar RS, Anjali CH, Mukherjee A, Chandrasekaran N (2012) Biodegradable polymer based encapsulation of neem oil nanoemulsion for controlled release of Aza A. Carbohydr Polym 90:1750-1756.
[Crossref] [Google Scholar] [PubMed]
- Dailey OD (2004) Volatilization of alachlor from polymeric formulations. J Agric Food Chem 52:6742-6746.
- Wu J, Chen J, Zhou Y, Nie WY, Cheng XM (2007) Structure characterization and insecticidal activity tests of natural pyrethrin and abermectin nanocapsules. Pestic 46:672.
- Yang FL, Li XG, Zhu F, Lei CL (2009) Structural characterization of nanoparticles loaded with garlic essential oil and their insecticidal activity against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J Agric Food Chem 57:10156-10162.
[Crossref] [Google Scholar] [PubMed]
- Adak T, Kumar J, Dey D, Shakil NA, Walia S (2012) Residue and bio-efficacy evaluation of controlled release formulations of imidacloprid against pests of soybean (Glycine max). J Environ Sci Health, Part B 47:226-231.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi