Research Article, J Nanomater Mol Nanotechnol Vol: 12 Issue: 3
Synthesis and Characterization of Zinc Oxide Nanoparticles by Sol Gel Method
Monis Bin Abid1*, Aisha Shamim2, Tariq Mahmood3, Jahangeer Khan4 and Zaheer Ahmad2
1Department of Chemistry, University of Prince Mugrin, Medina Al Munawara, Saudi Arabia
2Department of Chemistry, University of Wah, Wah Cantt, Pakistan
3Department of Nano Sciences and Technology, Quaid-i-Azam University, Islamabad, Pakistan
4Department of Chemistry, Soochow University, Funsome, China
*Corresponding Author:
- Monis Bin Abid
Department of Chemistry,
University of Prince Mugrin,
Medina Al Munawara,
Saudi
E-mail: Arabia; monisbinabid92@gmail.com
Received date: 03 August, 2020, Manuscript No. JNMN-23-16678;
Editor assigned date: 06 August, 2020, PreQC No. JNMN-23-16678 (PQ);
Reviewed date: 20 August, 2020, QC No. JNMN-23-16678;
Revised date: 18 August, 2023, Manuscript No. JNMN-23-16678 (R);
Published date: 15 September, 2023, DOI: 10.4172/2324-8777.1000363
Citation: Abid MB, Shamim A, Mahmood T, Khan J, Ahmad Z (2023) Synthesis and Characterization of Zinc Oxide Nanoparticles by Sol Gel Method. J Nanomater Mol Nanotechnol 12:3.
Abstract
The under-consideration study focuses on synthesis and characterization of Zinc Oxide (ZnO) nanoparticles. Nanosized zinc oxide powder was successfully synthesized using a simple and low cast sol-gel method. This method is environment friendly requiring no expensive chemicals and is time saving. The sol-gel method was accompanied by the formation of precipitates which were dried and calcined at 550? to get zinc oxide nanoparticles. The synthesized nanopowder was characterized by X-Ray Diffraction (XRD), Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDX)
Keywords: Synthesis, Zinc Oxide (ZnO), Nanoparticles, Sol-gel method, Energy dispersive spectroscopy
Introduction
Nanotechnology is a revolutionary field that deals with fabrication and applications of nanomaterials. Nanoparticles-particles of the size of (<100 nm) have attracted much of the focus of the scientific research due to their outstanding properties like large volume/surface ratio, surface tail ability and multifunctionality and vast applications. The particles which are made up of a few hundred atoms are used in scratchproof eyeglasses, crack resistant paints, anti-graffiti coatings for walls, transparent sunscreen, stain repellent fabrics, self-cleaning windows and ceramic coatings [1]. The nanoparticles especially the ZnO NPs are being used in industries for producing different products like rubber and paint. Moreover, they are especially conducive as anticancer and antibacterial. Metallic nanoparticles mostly possess unusual characteristics like large surface energies, quantum confinement, plasmon excitation, optical and magnetic properties. Oxides of nano particle size have unique properties and are widely used for solar energy conversion, catalysis, varistors and gas sensors [2].
Zinc oxide is one of the most interesting metallic nanoparticles found in nature as mineral called zincite. It has a typical wurtzite hexagonal structure and is a semiconductor material with a lot of desirable properties such as large binding energy, large range of excitation energy and controlled electrical conductivity [3]. Different methods are used in the synthesis of ZnO nano powder viz., sol gel method, hydrothermal or solvothermal method, micro-emulsion method, precipitation method and physical vapor depositio, laser ablation, chemical vapor deposition, electrochemical deposition, combustion, vapor transport synthesis, polyol synthesis pulsed laser deposition, sputtering, oxidation of metallic zinc powder, chemical bath deposition, soft template sol-gel method, arc plasma method, thermal evaporation and pechini-polymer complex method. Among all sol-gel method is a very attractive synthesis method and has several advantages like easy synthesis and control on chemical composition [4].
The ZnO nanoparticles are used in various products like medicine cosmetics, rubber and solar cells. Zinc is an essential element for human health and ZnO is generally documented as safe material by FDA are ceramics, glass, lubricants, paints, ointments, plastics, adhesives, sealants, ferrites, fire retarders, in nano lasers and semiconductor material. Zinc oxide NPs are used in biomedicine like biomedical imaging, drug delivery, gene delivery and bio sensing. Zinc oxide nanoparticles are very much important due to their utilization in gas sensors, biosensors, cosmetics, drug-delivery systems and so forth [5]. Zinc oxide Nanoparticles (ZnO NPs) also have remarkable optical, physical and antimicrobial properties and therefore have great potential to enhance agriculture. The biosynthesized zinc oxide nanoparticles exhibit strong antimicrobial behavior against bacterial and fungal species when employing the agar diffusion method. Synthesized ZnO nanoparticles exhibit anticancer activity against Daltons Lymphoma Ascites (DLA) cells as well as antimicrobial activity against some bacterial and fungal strains.
The objective of present work is to synthesize zinc oxide nanoparticles by sol-gel method. This is simple and cost-effective route because of use of inexpensive and few starting materials. By controlling structure, calcination temperature and pH value we synthesized pure ZnO NPs. There are various advantages of using solgel method such as homogeneous mixing, good crystallinity and sharp size distribution of nanoparticles [6].
Due to its unique properties, such as anti-corrosion, anti-bacterial, having low electron conductivity and having superior heat resistance, zinc oxide plays a significant role in modern industry. So, the goal of this project is to synthesise zinc oxide nanostructures using the sol-gel process in the most useful methods possible and to characterise the nanostructures. The simplest approach is the sol-gel one, and it may be used to adjust particle size and morphology by carefully monitoring reaction conditions. Zinc acetate dehydrate (Zn(CH3COO)2.2H2O) was utilised as a precursor, ethanol (CH2COOH) was used as the solvent, sodium hydroxide (NaOH) and distilled water were used as the media in the sol gel process to create ZnO nanoparticles. Nanoparticles analyzer, XRD, EDX and FESEM were used to characterise ZnO nanoparticles. The results of the EDX evaluation demonstrate the high purity of the ZnO nanoparticles, which have a 55.38% zinc and 44.62% oxygen content.
The oxygen and zinc peaks in the XRD result spectrum are the most prominent and show how crystallinity is present in nature. FESEM images demonstrate the rod-like structure of synthesised ZnO. The produced ZnO nanoparticles are uniform in size and homogeneous, which is commensurate with the good crystallinity shown by the XRD results. Sol-gel synthesis of ZnO nanoparticles in the 81.28 nm to 84.98 nm nanosize range was achieved. A recently developed study field that receives a lot of attention is the synthesis of metal nanoparticles with particular characteristics. For the synthesis of these materials, a number of techniques have been proposed. These include chemical vapour condensation, arc discharge, hydrogen plasma-metal reaction and laser pyrolysis in the vapour phase, microemulsion, hydrothermal, sol-gel, sonochemical and microbial processes in the liquid phase and ball milling in the solid phase. Metal nanoparticles' production processes have a significant impact on their characteristics. Metal oxides come in a wide diversity and they have a wide range of characteristics and potential uses. Due to its generally bio-safe and biocompatible composition, zinc oxide is an excellent material for use in sensors and transducers. In addition, nanostructured metal oxide possesses. Because of their catalytic, anti-corrosion, anti-bacterial and ultraviolet filtering characteristics, ZnO nanopowders are in high demand and employed extensively in industry. They have primarily been utilised recently as an ultraviolet-resistant ingredient in sunscreens. Electrophotography, photoprinting, capacitors, protective coatings, anti-microbial and conductive thin-films in LCDs, solar cells and blue laser diodes are a few further uses for zinc oxide nanopowder [7].
Materials and Methods
In present study ZnO NPs were synthesized by using chemical (solgel) method. This study was carried out at nanoscience and technology department, national centre for physics, QAU Islamabad and department of chemistry, university of Wah, Wah Cantt. These nanoparticles were characterized by using X-Ray Diffraction (XRD), Scanning Electron Microscopy (SEM) and Electron Dispersive X-ray spectroscopy (EDX). During this work all chemicals were purchased from local market of sigma-aldrich. These were AR-grade and there was no need of further purification. The chemicals used were, Zinc Chloride (ZnCl2) and 0.5 M sodium hydroxide. We used deionized water throughout the experiment [8].
Chemical synthesis
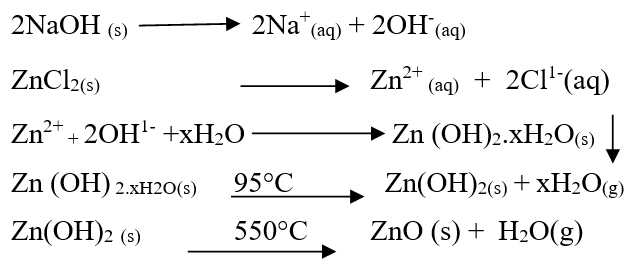
In this method aqueous solution of ZnCl2 was prepared by dissolving 3 g-4 g of salt in 100 ml of deionized water. We stirred the solution to dissolve the salt completely [9]. The salt solution was titrated by adding 0.5 M NaOH drop wise from burette with constant stirring. Frequently we checked the pH. The precipitates were formed when pH was 11. The colour of precipitates of zinc was white [9]. These precipitates were washed 4-5 times with de-ionized water and then were dried at 95°C to remove moisture. Dried precipitates were calcined at 550°C for three hours in a muffle furnace. The calcined material was grinded by mortar and pistil and sample was prepared [10]. Main reactions during procedure can be summarized as follows:
Characterization
We analyzed nanoparticles by using XRD model D8 Advance Bruker X-Source Copper (anode). The samples were characterized by XRD and their sizes were noted in nanometer. The synthesized NPs were also characterized by SEM performed on SEM, TESCAN, VEGA3 placed at advanced energy and material lab NUST. The EDX was done on EDX Oxford placed at fracture mechanics and fatigue lab, mechanical engineering department, UET Taxila [11].
Results and Discussion
X-Ray Diffraction (XRD) study
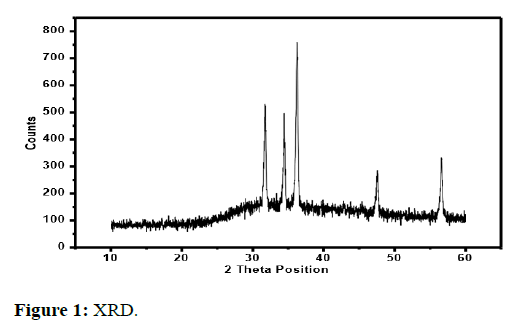
The results of the X-ray diffraction method are discussed below in Figure 1.
Spectrum of ZnO
XRD spectrum of chemically synthesized ZnO NPs show diffraction peaks at 31°, 34°, 36°, 47° and 56°. Sharp peaks show good crystallite growth and the position of peaks indicate the formation of pure ZnO nanoparticles. These values were also matched with JCPDF# 36-145 [12].
Scanning Electron Microscopy (SEM)
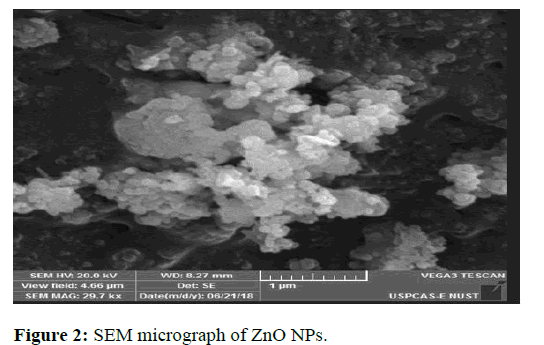
Scanning electron microscopy offers several advantages to determine size, shape and morphology of nanoparticles. The dry powder is placed on a holder and coated with gold. The surface of powder is then scanned with fine beam of electrons for analysis. Variety of signals is generated and reveals information related to the texture, composition, crystalline structure of material (Figure 2) [13].
The SEM micrographs of ZnO NPs clearly show beautiful white evenly sized crystals with hexagonal morphology. The average size of particles is 66 nm. Intense agglomeration in case of ZnO NPs is due to large surface energy of NPs [14].
Energy Dispersive X-ray spectroscopy (EDX)
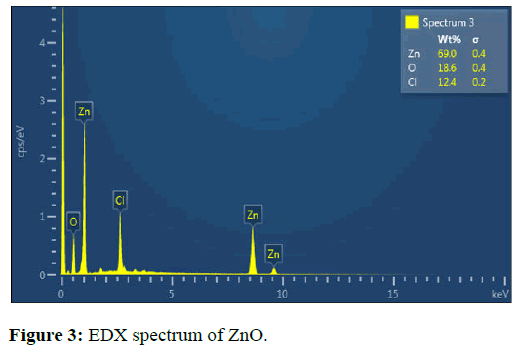
The EDX study showed the elements Zn and O. The Zn content was 69% while O content was 18.6%. The EDX results indicated that ZnO NPs were pure with only traces of impurities (Figure 3) [15].
Conclusion
The ZnO NPs were successfully fabricated via sol-gel method using zinc chloride and sodium hydroxide as main materials. The results indicated the formation of pure ZnO NPs without any impurity. The morphological study showed nano range of particles and elemental analysis successfully traced Zn and O elements. Sol-gel method is cheap and easy method and results in the formation of ZnO NPs with hexagonal morphology. The resultant nanoparticles were highly pure. Zinc oxide shows promising signs in the field of nanotechnology, UV detectors, nanoscale detectors and actuators. It would replace silicon in chip fabrication. The ZnO has bright future because of its dual semiconductor and piezoelectric properties.
Acknowledgement
We acknowledge the provision of services of NS and TD, NCP, QAU Islamabad.
References
- Li X, Xu H, Chen ZS, Chen G (2011) Biosynthesis of nanoparticles by microorganisms and their applications. J Nanomater 2011: 1-6.
- Chokriwal A, Sharma MM, Singh A (2014) Biological synthesis of nanoparticles using bacteria and their applications. Am J Pharm Tech Res 4: 38-61.
- Shamim A, Mahmood T, Abid MB (2019) Biogenic synthesis of Zinc Oxide (ZnO) nanoparticles using a fungus (Aspargillus niger) and their characterization. Int J Chem 11: 119.
- Kumar H, Venkatesh N, Bhowmik H, Kuila A (2018) Metallic nanoparticle: A review. Biomed J Sci Tech Res 4: 3765-3775.
- Al Abdullah K, Awad S, Zaraket J, Salame C (2017) Synthesis of ZnO nanopowders by using sol-gel and studying their structural and electrical properties at different temperature. Energy Proc 119: 565-570.
- Savi GD, Bortoluzzi AJ, Scussel VM (2013) Antifungal properties of zinc‐compounds against toxigenic fungi and mycotoxin. Int J Food Sci Technol 48: 1834-1840.
- Krol A, Pomastowski P, Rafinska K, Railean-Plugaru V, Buszewski B (2018) Corrigendum to zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv Colloid Interface Sci 254: 100.
[Crossref] [Google Scholar] [PubMed]
- Mahamuni PP, Patil PM, Dhanavade MJ, Badiger MV, Shadija PG, et al. (2019) Synthesis and characterization of zinc oxide nanoparticles by using polyol chemistry for their antimicrobial and antibiofilm activity. Biochem Biophys 17: 71-80.
[Crossref] [Google Scholar] [PubMed]
- Farahmandjou M, Jurablu S (2014) Co-precipitation synthesis of Zinc Oxide (ZnO) nanoparticles by zinc nitrate precursor. Int J Bio Inor Hybr Nanomater 3: 84.
- Agarwal H, Kumar SV, Rajeshkumar S (2017) A review on green synthesis of zinc oxide nanoparticles-An eco-friendly approach. Res Effic Technol 3: 406-413.
- Hasanpoor M, Aliofkhazraei M, Delavari HJ (2015) Microwave-assisted synthesis of zinc oxide nanoparticles. Proc Mater Sci 11: 320-325.
- Sabir S, Arshad M, Chaudhari SK (2014) Zinc oxide nanoparticles for revolutionizing agriculture: Synthesis and applications. Sci World J 2014: 925494.
[Crossref] [Google Scholar] [PubMed]
- Khan MF, Ansari AH, Hameedullah M, Ahmad E, Husain FM, et al. (2016) Sol-gel synthesis of thorn-like ZnO nanoparticles endorsing mechanical stirring effect and their antimicrobial activities: Potential role as nano-antibiotics. Sci Rep 6: 27689.
[Crossref] [Google Scholar] [PubMed]
- Delice S, Isik M, Gasanly NM (2019) Traps distribution in sol-gel synthesized ZnO nanoparticles. Mater Let 245: 103-105.
- Naveed Ul Haq A, Nadhman A, Ullah I, Mustafa G, Yasinzai M, et al. (2017) Synthesis approaches of zinc oxide nanoparticles: The dilemma of ecotoxicity. J Nanomater 2017.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi