Research Article, Dent Health Curr Res Vol: 5 Issue: 1
The Cytotoxic Effect of Titanium Oxide Surface Modified Orthodontic Stainless Steel Wires
Sameer Ahmad Malik*, Laxmikanth SM, Ramachandra CS, Shetty S and Reddy VS
Department Orthodontics and Dentofacial Orthopedics, AECS Maaruti College of Dental Sciences and Research Centre, Bangalore, India
*Corresponding Author : Sameer Ahmad Malik
Post Graduate MDS Student, Department of Orthodontics and Dentofacial Orthdopedics, AECS Maaruti College of Dental Sciences and Research Centre, Bangalore-560076, India
Tel: (+91) 8553340994
E-mail: malik11sameer@gmail.com
Received: 23 November 2018 Accepted: 03 December 2018 Published: 10 December 2018
Citation: Malik SA, Laxmikanth SM, Ramachandra CS, Shetty S and Reddy VS (2019) The Cytotoxic Effect of Titanium Oxide Surface Modified Orthodontic Stainless Steel Wires. Dent Health Curr Res 5:1. doi: 10.4172/2470-0886.1000137
Abstract
Background: The fixed orthodontic mechano therapy has been associated with white spot lesions and plaque accumulation. Titanium oxide (TiO2) is a compound that possesses clinically significant anti-microbial action especially against lactobacillus and streptococci. However the safety of the TiO2 coated wires has still not been tested. In this study, we evaluated the cytotoxic effects of TiO2 coated stainless steel orthodontic wires. To assess the same, we used A549 cells as experimental cell line. Cells were categorized into 4 groups (n=6/group): cellular control group, represented by the cell growth; negative control group (stainless steel wire) positive control group (hydrogen peroxide), experimental group (titanium oxide coated stainless steel wires). The cultures were carried out either on 6 well plates or 96 well plates and pictures were captured using a microscope or assessed by MTT assay.
Results: MTT assay revealed no cytotoxic effect by TiO2 coated wires as compared to control. Similarly, structural assessment of cellular morphology and nuclear membrane structure further showed no change in toxic effects of TiO2 coated SS wires on the cells.
Conclusion: Despite the limitations of this study, we demonstrated that titanium oxide coated wires had no cytotoxic effects like uncoated stainless steel wires and that there is a strong demand for long-term studies of TiO2 coated wires and brackets, with a strong focus on the cytotoxic properties, concentrations and exposure times, to make the desired applications for TiO2 coated wires safe and suitable for orthodontic use.
Keywords: Titanium oxide coated stainless steel orthodontic wires, A549, Stainless steel orthodontic wires, Sputtering
Introduction
The fixed orthodontic mechano therapy has been associated with white spot lesions and plaque accumulation, the reason for this being the bacterial adherence and accumulation around the brackets [1] and wires placed onto the tooth surfaces.
Titanium oxide is a compound that possesses clinically significant anti-microbial action, especially against lactobacillus and streptococci [2,3]. The titanium oxide’s antibacterial effect is based on its photo catalytic property [4]. The effectiveness of titanium oxide surface modified stainless steel brackets in reducing the bacterial adherence and the accumulation of bacteria around these brackets and wires has been established [2-4].
The Titanium oxide as a compound has also been associated with having certain cytotoxic effects in human and other mammalian cells [5,6]. A widespread application of titanium oxide nanoparticles (TiO2 NPs) raises the question about the safety of their use in the context of potential occupiers, environmental and intentional exposure of humans and biota. TiO2 NPs easily enter the body through inhalation, cross blood–brain barrier and accumulate in the brain, especially in the cortex and hippocampus [7]. Studies showed that TiO2 NPs exposure resulted in reactive oxygen species production, activation of signaling pathways involved in inflammation and cell death, both in vitro and in vivo [7]. Moreover, it has been shown that all titanium oxide nanoparticle-exposed cell lines displayed reactive oxygen species (ROS) generation [8]. Macrophage-like THP-1 and HPMEC-ST1 6R microvascular cells were sensitive to endogenous redox changes and underwent apoptosis, but not alveolar epithelial A549 cells [8]. In the same study the Genotoxic potential of titanium dioxide nanoparticles was investigated using the activation of γH2AX, activation of DNA repair proteins and cell cycle arrest wherein a persistent DNA damage was persistent and activation of DNA repair pathways was observed. However, we believe that the cytotoxic behavior of free NPs and bound particles might be different in its nature and intensity.
Furthermore, a study was conducted to determine if the biocompatibility of Stainless steel would be improved by modifying its surface with titanium. Therefore, they modified surface of stainless steel by titanium coating and micro-arc oxidation processed at 230 V, which also generated TiO2 on the surface. It was concluded that these surfaces modified stainless steel enhanced the biocompatibility compared to machine stainless steel [9]. In the current study we modified stainless steel orthodontic wires by coating them with titanium oxide, on the assumption the titanium oxide particles are adherent to the metal surface rather than in its free NP state (Figures 1-5).
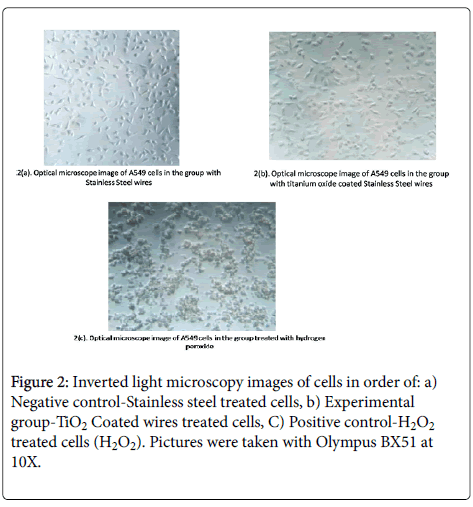
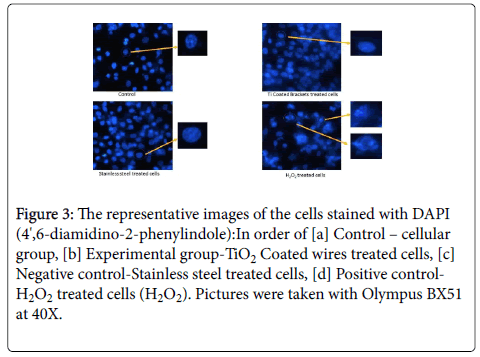
Figure 3: The representative images of the cells stained with DAPI (4',6-diamidino-2-phenylindole):In order of [a] Control – cellular group, [b] Experimental group-TiO2 Coated wires treated cells, [c] Negative control-Stainless steel treated cells, [d] Positive control- H2O2 treated cells (H2O2). Pictures were taken with Olympus BX51 at 40X.
Nowadays there are many studies on the biocompatibility of orthodontic materials because this is a real concern for clinicians, who do not want to place orthodontic appliances with a risk of adverse toxic effects in their patients. Till now no study has been undertaken to evaluate the cytotoxic effect of titanium oxide surface modified stainless steel wires. This study intended to evaluate and find out the toxic effect of these wires on the human cells: cellular behavior and viability and the relative patient safety factor in using these wires.
Materials and Methods
Preparation of photocatalytic titanium dioxidecoated orthodontic wires
Surface modification of stainless steel orthodontic wires (OPTIMA 19*25) with TiO2 were carried out using Tecport Sputter Coater by sputtering method in Indian Institute of Science, Bangalore.
Sputtering processes remove surface atoms or molecular fragments from a solid cathode (target) by bombarding it with positive ions from an inert gas (argon) discharge and deposit them on the nearby substrate to form a thin film [3].
In the present study, sputtering was carried out on stainless steel orthodontic wires (substrate) using Titanium as the target. Plasma generated inside the vacuumed chamber ejected surface atoms from the titanium target, which were sputtered onto sputtering was conducted for a period of 20 min. All wires were sputtered at the same time to achieve a thin and uniform coating of titanium. The sputtered titanium was further oxidized in the stainless steel wires (substrate). The distance between the substrate and the target was kept constant at 7 cm, and an ambient environment inside an open air furnace at 500°C for 5 h to provide a uniform coating of TiO2 on the stainless steel orthodontic wires.
Evaluation of cytotoxicity
This is an in vitro cytotoxicity test. Tests were performed in cell cultures lineage A549 Cell Line human lung carcinoma (ATCCAmerican Type Culture Collection) to evaluate the response rates, determined by MTT (3-(4,5-Dimethylthiazol-2-Yl)-2,5- Diphenyltetrazolium Bromide) assays.
The specimens were divided into 4 groups (n 6): cellular control group, represented by the cell growth; negative control group (stainless steel wire), whose material does not produce a cytotoxic response [10] positive control group (hydrogen peroxide), whose material is highly cytotoxic; experimental group (titanium oxide coated stainless steel wires).
Cells were manipulated in the laboratory of cellular and molecular biology at the Defense Institute of Physiology and Allied Sciences, Defense Research and Development Organization (DRDO), New Delhi, India. Cells were defrosted and cultured in Dulbecco modified eagle medium supplemented with 10% bovine fetal serum, 100 U per milliliter of penicillin, 100 mg per milliliter of streptomycin, and 50 mg per milliliter of gentamycin (complete Dulbecco modified eagle medium) in culture plates. The cells were incubated at a temperature of 37°C inan incubator (Thermo Scientific™) containing 5% carbon dioxide. After confluence of cells was obtained, the cells were removed by enzymatic action by using 0.1% trypsin ethylenediaminetetraacetic acid (Sigma-Aldrich Corporation, St Louis, Mo) and counted in a Neubauer chamber (Optik Labor, Friedrichsdorf, Germany). The suspension was added to plates of 96 wells, in 250-mL increments, with a density of 3000 cells per well. Finally, the cultures containing the specimens were again incubated for 24 hours. After the 24-hour incubation period, the plates were analyzed on an inverted light microscope (Olympus) with a 10X objective. The qualitative analysis was based on the characteristics of cell proliferation, growth, morphology, and adhesion. Cell viability was evaluated by the MTT assay (Sigma-Aldrich Corporation, St Louis, Mo), which is based on the ability of the mitochondrial enzyme succinate dehydrogenase to convert the yellow water-soluble tetrazolium salt (MTT) into formazan crystals in metabolically active cells. This water-insoluble, dark-blue product is stored in the cytoplasm of cells and is soluble afterward, generating a blue color [11]. After 24 hours, 200 mL of MTT was added to each well of the plate, followed by 4 hours of incubation at 37°C and 5% carbon dioxide. Then the medium was removed, and formazan crystals were dissolved with 120 mL per well of dimethyl sulfoxide (Sigma-Aldrich Corporation, St Louis, Mo), generating a blue color. Optical densities were measured at 570 nm in an ELISA reader, and cell viability was calculated according to the following formula:
Cell viability%=optical density of test group/optical density of cellular control group* 100
Cell morphology and nuclear structure evaluation: Cells were platted on 24 well tissue culture plates (Corning) at 3 × 105 cells each well. 6 wells were assigned to each group. Grouping and specimen treatment was done as mentioned above. Images were captured using Olympus inverted microscope at 10 × 0. 5 images were captured per sample and 30 per group to access changes in cellular morphology and integrity.
Nuclear morphology was evaluated by DAPI staining. Briefly 3 × 105 cells were plated on coverslips 24 well tissue culture plate and post treatment as mentioned above, cells were serum starved for 12 hours and then wells were washed with wash buffer (phosphate buffer saline, PBS, pH 7.4). Following washing, cells were fixed in ice-cold methanol for 5 min and then washed with PBS followed by staining with DAPI for 5 minutes. Post DAPI staining, cells were again washed 3 times with PBS and then coverslips were mounted on cells and visualized under Olympus BX51 at 40X. For each group 25 images were captured for assessment of nuclear structure.
Statistics
The collected data were analyzed with IBM SPSS software (version 22). To describe the data, descriptive statistics—means and standard deviations—were used. To find the significant difference between the groups one way ANOVA and Post hoc Tukey HSD test was used. In all these statistical tools, the probability value of 0.05 was considered to be significant.
Results
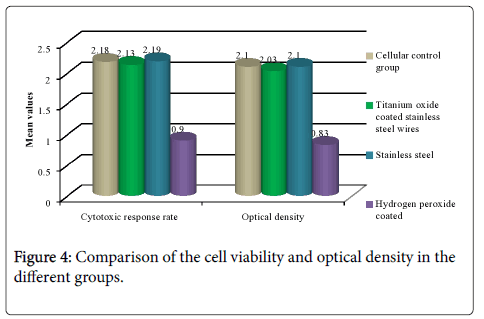
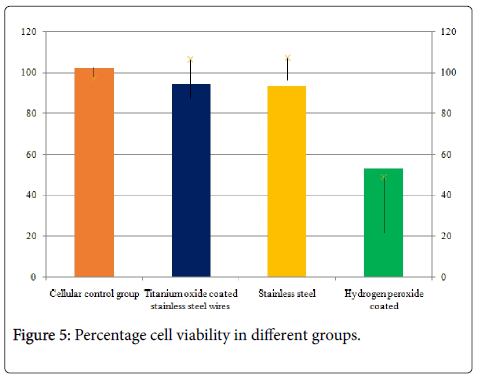
Titanium oxide coated orthodontic stainless steel wires did not affect cellular viability, to evaluate cell viability we performed MTT assay on A549 cells. Grouping of samples was done as 4 groups (n 6): cellular control group, represented by the cell growth; negative control group (stainless steel wires), whose material does not produce a cytotoxic response [10] positive control group (hydrogen peroxide), whose material is highly cytotoxic; experimental group (titanium oxide coated stainless steel wires). When the comparison of the cell viability was done it showed a significant difference between all the groups with almost similar response seen in Stainless steel and cellular control group followed by titanium oxide coated wires. Hydrogen peroxide showed the least cell viability (Table 1 and Figure 4). However when the inter group comparisons were done it was seen that there was a significant difference between titanium oxide coated wires and other groups (Tables 2 and 3). When the comparison of the optical dentistry was done it showed a significant difference between all the groups with similar mean values seen in Stainless steel and cellular control group followed by titanium oxide coated wires. Hydrogen peroxide coated wires showed the least cytotoxic response (Table 2, Figure 4). However when the inter group comparisons were done it was seen that there was a significant difference between titanium oxide coated wires and other groups (Table 4). Titanium oxide coated wires or stainless steel wires showed no observable change in cellular viability as compared to cellular control group. However, a significant decrease in cellular viability of hydrogen peroxide group was approximately 67% as compared to control (Figure 5).
| Groups | Mean ± SD | F | p |
|---|---|---|---|
| Cellular control group | 2.18 ± 0.04 | 89.256 | 0.00* |
| Titanium oxide coated stainless steel wires | 2.13 ± 0.13 | ||
| Stainless steel | 2.19 ± 0.10 | ||
| Hydrogen peroxide | 0.90 ± 0.27 | ||
| *Statistically significant, p<0.05 | |||
Table 1: Comparison of the cell viability in the different groups.
| Groups | Mean ± SD | F | p |
|---|---|---|---|
| Cellular control group | 2.10 ± 0.04 | 85.111 | 0.00* |
| Titanium oxide coated stainless steel wires | 2.03 ± 0.14 | ||
| Stainless steel | 2.10 ± 0.09 | ||
| Hydrogen peroxide coated | 0.83 ± 0.27 | ||
| *Statistically significant, p<0.05 | |||
Table 2: Comparison of the optical density in the different groups.
| Groups | Mean difference | p | |
|---|---|---|---|
| Cellular control group | Titanium oxide coated stainless steel wires | 0.053 | 0.94 |
| Stainless steel | -0.011 | 0.99 | |
| Hydrogen peroxide coated | 1.280 | 0.00* | |
| Titanium oxide coated stainless steel wires | Stainless steel | -0.064 | 0.90 |
| Hydrogen peroxide coated | 1.227 | 0.00* | |
| Stainless steel | Hydrogen peroxide coated | 1.292 | 0.00* |
| *Statistically significant#Post hoc Turkey HSD, p#<0.05 | |||
Table 3: Inter group comparison cell viability.
| Groups | Mean difference | p | |
|---|---|---|---|
| Cellular control group | Titanium oxide coated stainless steel wires | 0.07 | 0.88 |
| Stainless steel | 0.00 | 1.00 | |
| Hydrogen peroxide coated | 1.26 | 0.00* | |
| Titanium oxide coated stainless steel wires | Stainless steel | -0.06 | 0.89 |
| Hydrogen peroxide coated | 1.19 | 0.00* | |
| Stainless steel | Hydrogen peroxide coated | 1.26 | 0.00* |
| *Statistically significant#Post hoc Turkey HSD, p#<0.05 | |||
Table 4: Inter group comparison of cytotoxic response rate by MTT.
Titanium oxide coated orthodontic stainless steel wires did not affect cellular morphology
To assess any epithelial/mesenchymal transition [EMT] and cytoskeletal changes leading to altered cellular morphology, we evaluated the change in cellular structure, integrity and viability under inverted microscopy. The pictorial representations of all groups has been shown in Figure 2 wherein a marked cell death can be seen in Figure 3©/peroxide group as compared to [a, b]. The pictures were captured using OlympusBX 51 inverted microscope at 10X.
Titanium oxide coated orthodontic stainless steel wires did not alter nuclear structure; the representative images of cells stained with DAPI (4',6-diamidino-2-phenylindole) as shown in Figure 3 demonstrate a marked structural change in nucleii of hydrogen peroxide group. However, we did not observe any structural change in nucleii of other groups. Hydrogen peroxide is used as a positive control group to induce cellular and nuclear damage. We observed nuclear membrane lysis and loss of nuclear membrane structural integrity in hydrogen peroxide group as shown in subset of Figure 3 [d]. Whereas, no such changes were observed in experimental group as compared to positive control group. The images were captured using Olympus BX51 at 40X and subsets were manually zoomed to 100X using Image J [NIH] software.
Discussion
The photocatalytic activity of illuminated TiO2 has been actively investigated in diverse areas such as water-treatment processes, air-cleaning agents, and antibacterial agents. Illuminated TiO2 in water with light at a wavelength less than 380 NM generates excess electrons in the conduction band (e sub) and positive ‘‘holes’’ in the valence band (hvb). At the TiO2 particle surface, the holes react with either adsorbed water or surface hydroxyl (OH) groups to form HO• radicals. The excess electrons in the conduction band react with molecular oxygen to form superoxide ions, which form more HO• radicals. In aqueous systems, the complete mineralization of many organic substances is possible when a sufficient HO• flux can be generated in situ. Therefore, suspended TiO2 particles have largely been used as efficient catalysts for the decomposition of organic contaminants [4].
Several attempts have been made to apply the photocatalytic activity of TiO2 to microorganisms [12-14] and same has been successfully achieved in the field of orthodontics by proving there is a significant antibacterial effect of titanium oxide against lactobacillus acidophilus which is one of the main colonizing bacteria in plaque around the orthodontic fixed appliances [3].
However, the cytotoxicity of titanium oxide is a concern for its safe use. Its cytotoxicity has been proved and disapproved in various studies under various clinical circumstances and under various differing variables [5,6,8,9]
It is commonly accepted that titanium alloying elements are biocompatible [15]. Perhaps the oxidation of titanium increases the cytotoxicity. Some researchers have monitored the cytotoxicity by evaluating the adhesion, proliferation, and metabolism of cells, such as 3T3, L929, and W138, and human fibroblasts and osteoblasts [16,17]. Our study was similar to the study of Grill et al. [16], who advocated the investigation of proliferation by microscopic analysis of cell growth and division and cell viability assessment by the MTT assay. According to our findings, no significant cell modifications indicate no cytotoxicity of titanium coated stainless steel wires used in orthodontics. Li et al. [15] hypothesized that cytotoxicity is linked to the ions released from the metals and established that a safe molybdenum ion concentration (below which the ion concentration is nontoxic) is 8.5 mg per liter [15]. So, by the extension of same principle we hypothesize that the concentration of release of titanium oxide coating was below the optimal toxic levels.
The limitation of our study is with respect to its sample size. So, it is essential to continue these studies to assess the biocompatibility of these coated wires or otherwise if found cytotoxic to any extent under any other lab conditions, that amount of cytotoxicity needs to be evaluated. However the results which we achieved in our study, if passed the test of time could open doors for the development of newer antibacterial orthodontic appliances without any fear of any cytotoxic risk to the patient.
Conclusion
We demonstrate for the first time, despite the limitations of this study, that titanium oxide coated wires had no cytotoxic effects like uncoated stainless steel wires. This suggests that TiO2 coated wires don’t possess a potential health risk and that there is a strong demand for long-term studies of TiO2 coated wires and brackets, with a strong focus on the cytotoxic properties, concentrations and exposure times, to make the desired applications for TiO2 coated wires and brackets safe and suitable for orthodontic use.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Some part of experimentation was at Defense Research and Development Organization [DRDO], New Delhi, India
Authors contributions
1. Sameer Ahmad Malik – corresponding author, conducted most of the research work, supervised the lab procedures and compiling the results and preparing the manuscript.
2. Laxmikanth SM – Inception of the idea of this research work. All research work done under his guidance.
3. Ramachandra CS – Designed and handling of the statistical data.
4. Shetty S – Contributed towards technical considerations in the sputtering of arch wires.
5. Reddy VS – Assisted in the lab procedures with certain thoughtful inputs on the cell culture growth and microscopy.
Funding
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- Gorelick L, Arnold MG, and Gwinnett AJ (1982) Incidence of white spot formation after bonding and banding. Am J Orthod 81: 93-98.
- Choi JY, Chung CJ, Oh KT, Choi YJ, Kim KH (2009) Photo catalytic antibacterial effect of TiO2 Film of TiAg on Streptococcus mutans. Angle Orthod 79: 528-532.
- Shah AG, Shetty PC, Ramachandra CS, Bhat S, Laxmikanth SM (2011) In vitroassessment of photo catalytic titanium oxide surface modified stainless steel orthodontic Brackets for antiadherent and anti-bacterial properties against Lactobacillus acidophilus. Angle Orthod 81: 6.
- Chun MJ, Shim E, Kho EH, Hwang HS (2007) Surface modification of orthodontic wires with photo catalytic titanium oxide for its anti-adherent and antibacterial properties. Angle Orthod 77: 483-488.
- Ze Y, Zheng L, Zhao X, Gui S, Sang X, et al. (2013) Molecular mechanism of titanium dioxide nanoparticles-induced oxidative injury in the brain of mice. Chemosphere 92: 1183-1189.
- Jaeger A, Weiss DG, Jonas L, Kriehuber R (2012) Oxidative stress-induced cytotoxic and genotoxic effects of nano-sized titanium dioxide particles in human HaCaT keratinocytes. Toxicology 296: 27-36.
- Retamoso LB, Luz TB, Marinowic DR, Machado DC, Menezes LMD, et al. (2012) Cytotoxicity of esthetic, metallic, and nickel-free orthodontic brackets: Cellular behavior and viability. Am J Orthod Dentofacial Orthop 142:70-74.
- Hanot-Roy M, Tubeuf E, Guilbert A, Bado-Nilles A, Vigneron P, et al. (2016) Oxidative stress pathways involved in cytotoxicity and genotoxicity of titanium dioxide (TiO2) nanoparticles on cells constitutive of alveolo-capillary barrier in vitro. Toxicology in vitro 33: 125-135.
- Lim YW, Kwon SY, Sun DH, Kim HE, Kim YS (2011) Improved biocompatibility of stainless steel implant by titanium coating and micro-arc Korea. 55th Annual Meeting of the Orthopaedic Research Society.
- Freitas MP, Oshima HM, Menezes LM, Machado DC, Viezzer C (2009) Cytotoxicity of silver solder employed in orthodontics. Angle Orthod 79: 939-944.
- Liu Y, Peterson DA, Kimura H, Schubert D (1997) Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem 69: 581-593.
- Ireland JC, Klostermann P, Rice EW, Clark RM (1993) Inactivation of Escherichia coli by titanium dioxide photocatalytic oxidation. Appl Environ Microbiol 59: 1668-1670.
- Cho M, Chung H, Choi W, Yoon J (2004) Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Res 38: 1069-1077.
- Ame´zaga-Madrid P, Silveyra-Morales R, Co´rdoba-Fierro L, Neva´ rez-Moorillo´n GV, Miki-Yoshida M, et al. (2003) TEM evidence of ultrastructural alteration on Pseudomonas photocatalytic TiO2 thin films. J PhotochemPhotobiol B 70: 45-50.
- Li Y, Wong C, Xiong J, Hodgson P, Wen C (2010) Cytotoxicity of titanium and titanium alloying elements. J Dent Res 89: 493-497.
- Grill V, Sandrucci MA, Basa M, Di Lenarda R, Dorigo E, et al. (1997) The influence of dental metal alloys on cell proliferation and the fibronectin arrangement in human fibroblast cultures. Arch Oral Biol 42: 641-647.
- Santos RL, Pithon MM, Martins FO, Romanos MT, Ruellas AC (2010) Cytotoxicity of latex and non-latex orthodontic elastomeric ligatures on L929 mouse fibroblasts. Braz Dent J 21: 205-210.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi