Research Article, Dent Health Curr Res Vol: 5 Issue: 2
The Inter-Relationship between Periodontal Disease and Diabetes Mellitus: A Review and Four Case Reports
Guadalupe Josefina Mikel Tostado1*, Caroline Chanussot Deprez1, Sara Gabriela Núñez Romo1, Maria Fernanda Estrada Viveros2 and José Daniel Carbente Fernández3
1General Hospital of Pemex de Veracruz, Miguel Angel De Quevedo S/N Esq. Raz Y Guzmán, Quinta María, 91859 Veracruz, Mexico
2University in Burlington, Burlington, Vermont, VT 05405
3Calz Simon Bolívar 339, Ignacio Zaragoza, 91910 Veracruz, Mexico
*Corresponding Author : Guadalupe Josefina Mikel Tostado
General Hospital of Pemex de Veracruz., Miguel Angel de Quevedo S/N, Corner Raz y Guzman, Colonia Formando Hogar, 91710, Veracruz, Mexico
Tel: +229-9892800
E-mail: guadalupe.josefina.mikel@pemex.com
Received: April 25, 2018 Accepted: December 29, 2018 Published: January 05, 2019
Citation: Tostado GJM, Deprez CC, Romo SGN, Viveros MFE, Fernández JDC (2019) The Inter-Relationship between Periodontal Disease and Diabetes Mellitus: A Review and Four Case Reports. Dent Health Curr Res 5:1. doi: 10.4172/2470-0886.1000141
Abstract
Diabetes is a risk factor for periodontal disease the same way periodontal disease is a risk factor for the progression of diabetes. These two diseases are correlated; periodontal disease can be favored by bacterial infection caused by an alteration of the immune system because of diabetes mellitus as well as chronic inflammation caused by periodontal disease leads to poor glycemic control in these patients. A review of the literature on the relationship between these two diseases is carried out. The case reported here illustrates the problem. To improve periodontal disease, it has been proven that the care of patients that includes a correct brushing technique and being aware of the condition of their oral health is effective. However, it is necessary to educate and guide patients with diabetes on the self-care of periodontal disease.
Keywords: Diabetes mellitus, Periodontal disease, Hyperglycemia
Abbreviations
INEGI: National Institute of Statistics and Geography, Mexico; DMPD: Diabetes Mellitus Periodontal Disease; TNFα: Tumor Necrosis Factor Alpha; Il 6: Interleukin 6; FEO: Fetor Ex Ore; AGE: Advanced Glycation End Products
Introduction
Oral pathologies include alterations of the periodontium like periodontal disease and manifests as gingivitis and periodontitis, affecting soft gingival tissues and periodontal-ligament attachment to
bone. Periodontitis and systemic diseases are interrelated.
Periodontitis causes destruction of tooth attachment to bone and may predispose to systemic disease which can precipitate accelerated periodontal destruction and tooth-loss [1-3].
Diabetes mellitus is a metabolic disease involving insulin affecting stable glycemia [4].
In 2017, adult worldwide prevalence was 8.8% and rising [5].
INEGI reports diabetes mellitus as the leader (15%) of cause-related deaths in Mexico. The National Institute of Public Health indicates 14% of adult Mexicans are diabetic and in over 60 years old, the prevalence is 30% [6].
Periodontopathic biofilm and resulting periodontal destruction, thrives in compromised immunity induced from diabetes, and reciprocally aggravates chronic inflammation which worsens glycemic control in diabetics [7].
The initial pioneer organisms Streptococcus oralis, S. mitis, S. mutans, S. gordonii, and S. sanguis; early and intermediate colonizing microbes facultative and Gram negatives, including Corynebacteria ochracea, Prevotella acnes, Porphyromonas gingivalis, Actinomyces naeslundii, A. Israelii, P. Loeschii, H. Parainfluenzae, C. sputegena, Prevotella denticola, and Vionella atypical species; major microbial organism late colonizers……Fusobacterium nucleatumand include Agrigatibacter actinomycetemcommitans (Aa), Black pigmented bacteroides( BPB), Prevotella intermedia (Pr-i), Tanarella forsythia (Tf), Porphyromonas gingivalis (Pg), Eubacterium spp. Treponema denticola, T. macrodentium/microdentium and others.
Significant differences were observed in subgingival microbiota between diabetic and nondiabetic subjects. Diabetic subjects presented higher percentages of total clones of TM7, Aggregatibacter, Neisseria, Gemella, Eikenella, Selenomonas, Actinomyces, Capnocytophaga, Fusobacterium, Veillonella and Streptococcus genera, and lower percentages of Porphyromonas, Filifactor, Eubacterium, Synergistetes, Tannerella and Treponema genera than nondiabetic individuals. Moreover, some phylotypes, such as Fusobacterium nucleatum, Veillonella parvula, V. dispar and Eikenella corrodens were detected significantly more often in diabetic subjects than in nondiabetic subjects [7].
Consequently diabetes is regarded as a risk factor for periodontitis and vice versa, Subjects with uncontrolled type-2 diabetes and chronic periodontitis presented significant dissimilarities in subgingival biodiversity compared with nondiabetic subjects [4,7-9].
Relationship between periodontal diseases and diabetes mellitus
The Diabetes-Mellitus/ Periodontal Disease (DMPD) relationship is widely acknowledged, but diabetes alone does not cause gingival disease, but favors the accelerated pathological modification process and aggravates clinical presentations when stagnated mature oral biofilm initiates periodontitis [10].
Gingivae of untreated diabetics become erythematous and edematous; some may be hypertrophied, typically with pain and suppuration oozing from gingival margins and inter-dental papillae [10]. This association in poorly controlled diabetics with increased gingivitis and bleeding is described frequently [11].
Aggressive gingivitis can progress to periodontal disease; this progression becomes problematic in diabetics, because of the faster progress, greater severity and higher prevalence of periodontal destruction when compared to non-diabetic patients. Periodontal disease compromises optimal mastication and this may leads to inadequate diets. A periodontal infection can affect the metabolic control of diabetes [12].
Diabetics frequently don’t tolerate removable dental prostheses due to alveolar bone loss and associated mucosal lesions [10].
The DMPD has many moderating and confounding influences at work. For example: poor metabolic control of glycemia; age; longer times of developing diabetes; later complications,[including increased antigenic action of glutamic acid decarboxylase, and increased production of IgG against Porphyromonas gingivalis; and increased levels of metalloproteinase in saliva, have all been putatively implicated and indicated in refractory and/or advanced periodontitis in diabetic patients [10].
The increased frequency of periodontitis in diabetics remains obscure but some patho-physiological changes are known.
Vascular changes
Microvascular changes occur in gingivae and alveolar mucosa of diabetics, are similar to those in other organs and tissues [9]. The fundamental structural change of small vessels is thickening of the basement membrane, characterized by accumulation of fibrillar material with collagen fibers. These changes include: the basement membranes of capillaries, narrowing of lumens and peri-endothelial thickening, accompanied by stasis in the microcirculation. With hyperglycemia basal membrane proteins of blood vessels don’t carry out enzymatic glycosylation; this causes physical changes that alter the structure, composition and permeability of the membranes. These alterations all influence the severity of periodontitis in diabetics, because they would render poor diffusion of oxygen, with inadequate elimination of metabolic waste, decrease leukocyte migration and cause poor diffusion of humoral factors [10].
Alterations in the oral microflora
Oral microflora is altered in diabetes mellitus. The predominant microorganisms vary from one study to another: gram-negative bacteria in general, Staphylococcus, fundamentally epidermidis, Capnocytophaga and anaerobic vibrios, A. actinomycetemcomitans and pigmented bacteroides, Prevotella intermedia , also P.gingivalis and W.recta [7].
Inadequate host response
Bacterial infection in diabetic patients may be contributed by a defect in the function of polymorphonuclear cells. A decrease in chemotaxis, adherence and phagocytosis in the peripheral leukocytes [13]. Despite this, the origin of the polymorphonuclear functional deficit is still unclear.
Diabetics with severe periodontitis have a reduced chemotaxis when compared with diabetics with medium periodontitis or with non-diabetics with medium or severe periodontitis [13].
Also the cellular immunity can in some cases influence the pathogenesis of the disease since in some cases it has a protective effect and in other cases an aggressive effect. In some studies, no significant differences have been seen in the function of the T. lymphocytes of patients with periodontitis. The determination of the precise role of the T-cell in periodontitis,at the present time, is difficult. It is therefore very important to examine the phenotype, cytokine production, and function of the T-cells.
It is possible that the mix of naive, activated, and memory T-cells, as well as the presence of Th-0, Th-1, andTh-2 cells, represents different phases of the disease. Since early "naive" T-cells are predominantly Th-0, the presence of these cells may indicate an early stage of the disease. Once the micro-flora associated with periodontitis is established, the bacteria induce the production of IL-l and activate T-cells to produce IL-2, which is indicative of a Th-1 phenotype. Thus, the early T-cell response would be of the Th-1type. The IL-2 could then activate other T-cells, including more Th-l cells, as well as Th-2 cells. A continued strong Th-l response would maintain anti-bacterial activity and limit the disease process. Enhanced Th-I activity would result in the recruitment of NK cells, killer cells, and CD8+ T-cells to the site, which may limit the disease. The presence of IFN-y and IL-2 messages indeed demonstrates the presence of Th-l cells. In contrast, if a Th-2 response (characterized by IL-4, IL-5, and IL-10 production) predominates, the cellular infiltrate would be characterized by increased B-cell development and increased antibody production. A marked increase in the numbers of plasma cells does indeed suggest enhanced Th-2 activity in the advanced periodontal lesion. Increased anti-bacterial antibody would eliminate bacteria, but if the antibodies produced were either protective for the bacteria or ineffective at eliminating the micro-organisms, the disease may progress. The presence of both Th-1 and Th-2 cells in the periodontal lesion indicates that both cell subsets are important in the disease process. Whether a Th-1 or a Th-2 phenotype predominates could possibly determine the outcome of the disease. Thus, the dichotomy between "protective" and "destructive" antibody production could well be the key in determining whether or not disease ensues [14].
Evaluating the complete system, diabetic patients with periodontitis showed significantly higher activity than non-diabetic patients, perhaps as an attempt to compensate for the weakened cellular immune response [13].
Increased glucose levels and resultant glycogenic end-products may alter the host response against bacterial infection [9]. Advanced glycation end products (AGE) are synthesized via the non-enzymatic glycation and oxidation of proteins, lipids and nucleic acids. The production is particularly enhanced in chronic hyperglycemia, as in diabetes mellitus. The formation of irreversible AGEs affects the tissues by compromising the physiologic and mechanical functions, as a result of defective constitution of the extracellular matrix components. Periodontitis is an inflammatory disease of microbial origin, resulting in devastation of the tooth supporting apparatus. This disease condition has severe implications in subjects with diabetes, since the tooth supporting tissues contain extracellular matrix targeted by AGE [15].
Inflammatory cytokines control the inflammatory process and generally remain in balance, however, in patients with diabetes mellitus, this balance is interrupted and hyperglycemia is associated with the severity of the periodontal condition. An increase in tumor necrosis factor alpha (TNFα) and interleukin 6 (IL 6) has been seen in diabetic patients with periodontitis [16].
4-Abnormal metabolism of collagen: this alteration would contribute to the periodontal disease progression and to the torrid healing of wounds, traits frequently observed in diabetic patients. A lower proliferation and cell growth, as well as a decrease in collagen synthesis by skin fibroblasts under conditions of hyperglycemia [17]. Other changes include: an increased collagenolytic activity of the gingival tissues in the group of diabetic patients, [18] a decrease in the synthesis of collagen by the fibroblasts of the gingiva and periodontal ligament, and an increase in the activity of the gingival collagenase [19,20].
High levels of glucose induce the activation of NF-KB, which in turn induces changes in the expression of periodontal ligament fibroblast genes, favoring neutrophil activation and bone resorption [21].
Four cases that illustrate periodontal disease and diabetes mellitus are presented
Case Report:A 65 year old male patient presented: he had been diagnosed diabetes mellitus and hypertension several years ago and has medical treatment for this two diseases.
On examination of oral cavity: he had good opening and had most of his permanent teeth.
Soft Tissues: they are inflamed (gingival margins were bleeding with inflamed inter-dental papillae).
The mucosa, uvula, tongue, cheeks, hard and soft-palate and floor of the mouth were all without pathological change, and within the range of health.
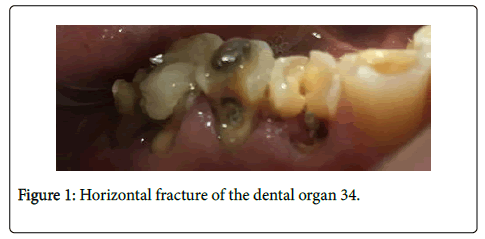
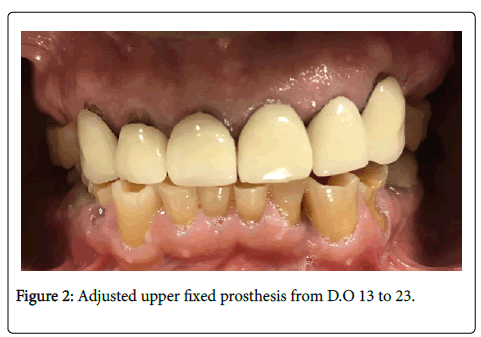
Teeth: The tooth 34 presents a horizontal fracture (Figure 1) and also an adjusted upper fixed prosthesis from tooth 13 to tooth 23 (Figure 2).
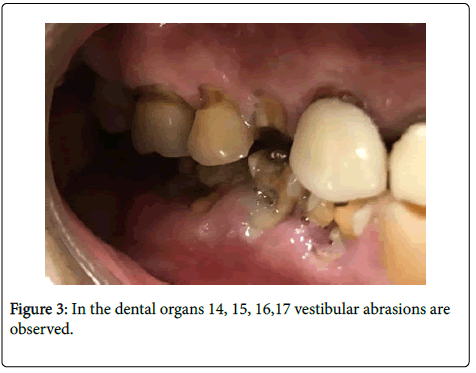
Tooth # 14: part of the root it exposed.
Tooth # 15 had a vestibular abrasion
Tooth # 16 has a mismatched amalgam
Tooth # 17 had a mismatched amalgam and a gingival recession.
Teeth # 14, 15, 16, 17 presented vestibular abrasions (Figure 3).
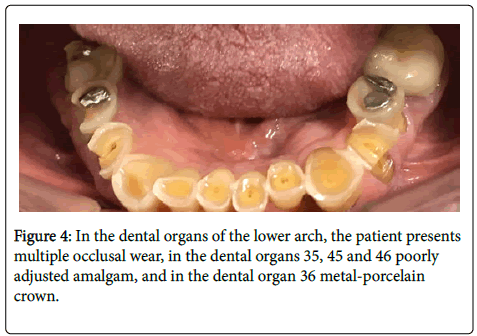
Tooth # 44 had a fracture, a gingival recession and radicular exposure.
Teeth # 35, 45 and 46 presented unsettled amalgam
Tooth # 36 restored with porcelain metal crown.
In the teeth of the lower arch, the patient presented multiple occlusal wear. In the tooth # 35, 45 and 46 poorly adjusted amalgam, and in the tooth # 36 metal-porcelain crown (Figure 4).
Case Report: A 70-year-old female patient presented: she was asymptomatic but after special investigations was diagnosed with diabetes and hypertension. On examination of the oral cavity the following was noted: good opening; she had most of her permanent teeth.
Soft tissues: the gingival margins were bleeding with inflamed inter-dental papillae.
The mucosa, uvula, tongue, cheeks, hard and soft-palate and floor of the mouth were all without pathological change, and within the range of health.
Teeth: She had abundant tartar on the lower teeth even though she performed regular oral hygiene.
She had a noticeable oral malodor that derived from a combination of ketotic pathological halitosis and fetor ex ore (FEO) from her decaying teeth, bleeding gums and poor prosthetic hygiene. She reports she uses her removable partial denture.
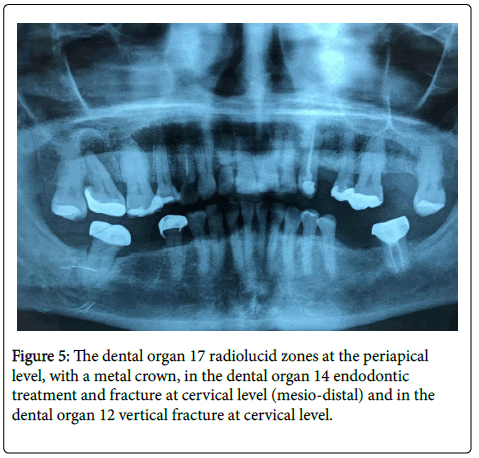
Tooth #12 had a vertical fracture at the cervical level.
Tooth # 14 has endodontic treatment secondary to caries, and showed recurrent decay with a vertical fracture inter-proximally, (mesio-distally). Intra-oral peri-apical radiographs showed a circumscribed radiolucent apical area.
Tooth # 17 was restored with amalgam. On Radiograph a Periapical area was present, which was not connected to the maxillary sinus. It shows grade I mobility
(#17 either had an amalgam or a crown; was the old amalgam part of the core? This is not clear)
Tooth # 18 had extensive caries which contributed to the FEO (Figure 5).
Case Report: A 45-year-old male patient presented with a history of diabetes Mellitus and arterial hypertension in medical treatment.
In oral exploration with dry lips, good opening and he had his permanent teeth.
Soft tissues: the gingival margins were bleeding with inflamed inter-dental papillae. The mucosa, uvula, tongue, cheeks, hard and soft-palate and floor of the mouth were all without pathological change, and within the range of health.
Teeth: Teeth # 11 and # 12 have caries and demineralization in cervical third with vertical fracture.
Tooth # 14 and tooth #15 had endodontic treatment and the roots remain with decay.
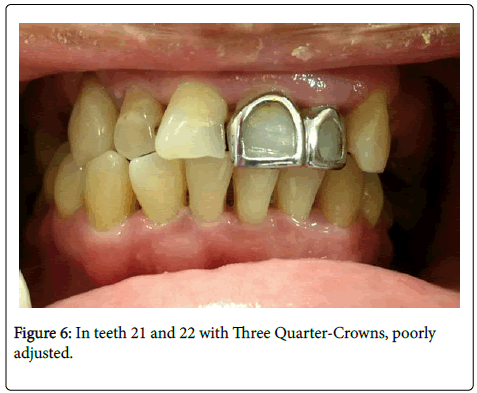
In teeth # 21 and # 22 had a three Quarter-Crowns (poorly adjusted) (Figure 6).
Tooth #25 and # 26 had caries.
Tooth # 27 had a poorly adjusted occlusal amalgam with microfiltration.
Tooth # 28 had caries in occlusal.
Teeth # 31, 32 and 33 had a gingival recession by palatal.
Tooth # 44 had occlusal caries in the gingival sulcus.
Tooth # 35 and # 36 had a poorly adjusted occlusal amalgam with microfiltration.
Tooth # 38 had an occlusal caries
Teeth # 41, 42, 43 and 44 had a gingival recession in palatine.
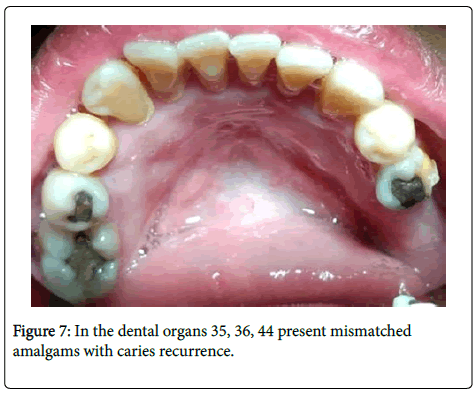
In the teeth #35, #36, #44 present mismatched amalgams with caries recurrence (Figure 7).
Tooth #45 had a coronary fracture, amalgam with microfiltration
Tooth # 48 had an occlusal caries.
Teeth # 37, #46 and #44 were absent.
Radiographic exploration shows generalized bone loss.
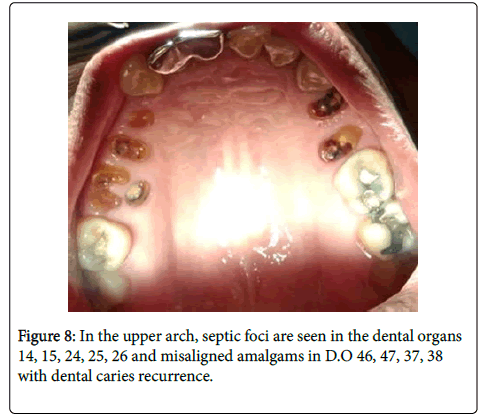
In the upper arch, septic foci are seen in the teeth #14, #15, #24, #25, #26 and misaligned amalgams in teeth # 46, #47, #37, #38 with dental caries recurrence (Figure 8).
Case Report: A 49 year female old patient presented; she was diagnosed diabetes Mellitus and received medical treatment.
On examination of the oral cavity: good opening of the mouth; she had most of her permanent teeth buth the middle line deviated 2.5 mm to the right, does not coincide with her sagittal axis.
Soft tissues: the gingival margins were bleeding with inflamed inter-dental papillae.
The mucosa, uvula, tongue, cheeks, hard and soft-palate and floor of the mouth were all without pathological change, and within the range of health.
Teeth: she had an orthodontic treatment (brackets) with orthodontic bands in teeth #16, #26, #36, #47.
Teeth # 31 and #41 had a diastema in the inferior centrals.
Tooth #48 with mobility class 3
Tooth #36 received treatment of the canal with poorly adjusted crown with filtration.
Right canine relationship class 1
Radiographically: generalized bone loss is observed , has resorption in the teeth 4.6, metal crown in teeth 3.6. Onlay inlay in teeth #15, # 25, # 27, #37, #47.
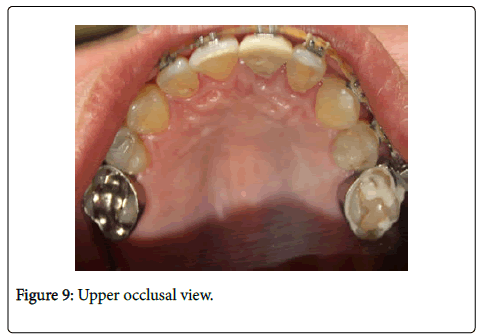
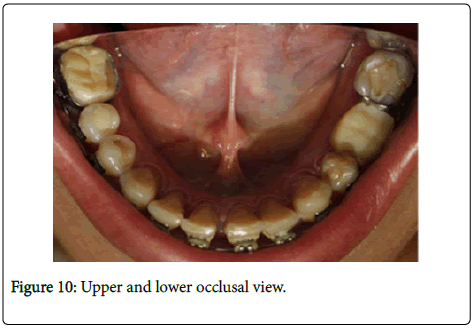
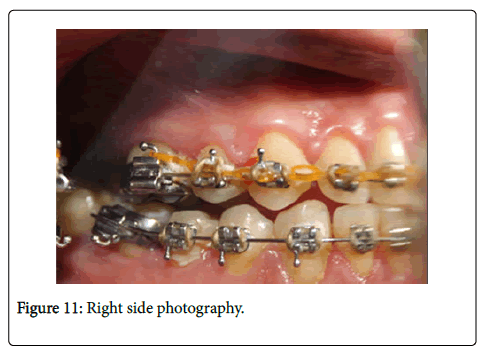
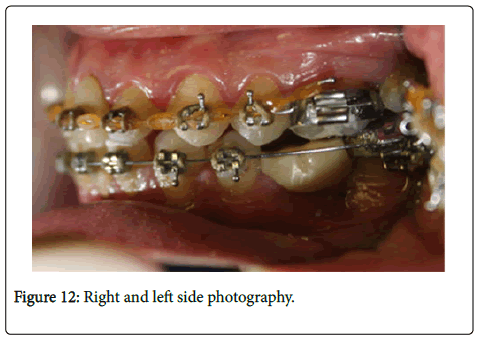
–In Figure 9 and Figure 10 we see upper and lower occlusal view and in Figure 11 and Figure 12- Right and left side.
In all patients the treatment of the periodontal disease included special cleaning to remove plaque and tartar deposits on the teeth and root surface.
All patients receive advices about dental brushing techniques and being aware of the condition of their oral health.
Conclusion
Patients suffering from periodontal disease improve dramatically with stringent oral hygiene.
Increased awareness with upgrading oral hygiene techniques and instructions, significantly improves oral health, general health and consequent glycemic control, in Type II diabetics.
References
- Carranza F (2004) Etiology of periodontal diseases. Periodontología clínica (9th edtn). McGraw-Hill Interamericana, México.
- Martinez AB (1998) Etiopatogenia de la enfermedad periodontal.En: tratado de odontología.T33. Trigo ediciones, Madrid, Spain.
- Sisto MP, Sisto LP, Felizola AD, Keiruz DT, Salas NL (2008) La enfermedad periodontal como riesgo de enfermedades sistémicas. Rev Cubana Estomatol 45: 1-9.
- Chávarry NG, Vettore MV, Sansone C, Sheiham A (2009) The relationship between diabetes mellitus and destructive periodontal disease:a meta analysis. Oral Health Prev Dent 7: 107-127.
- Janowitz A, Biagi L, Ito U (2018) Statista - the statistics portal for market data, market research and market studies
- Federacion Mexicana de Diabetes (2017) Estadísticas en México archivos.
- Casarin RCV, Barbagallo A, Meulman T, Santos VR, Sallum EA, et al. (2013) Subgingival biodiversity in subjects with uncontrolled type-2 diabetes and chronic periodontitis. J Periodontal Res 48: 30-36.
- Salvi GE, Carollo-Bittel B, Lang NP (2008) Effect of diabetes mellitus on periodontal and peri-implant conditions: update on association and risks. J Clin Periodontal 35: 398-409.
- Nishihara RNU, Tanabe DHN, Nakamura T, Okada Y, Nishida T, et al. (2017) A periodontal disease care program for patients with type 2 diabetes: a randomized controlled trial. J Gen Fam Med 18: 249-257.
- Blanco JJA, Villar BB, Martinez EJ, Vallejo PS, Blanco FJA (2003) Problemas bucodentales en pacientes con diabetes mellitus: índice gingival enfermedad periodontal. Med Oral 8: 233-247.
- Barreiro AD (1981) La diabetes en odontología. An Es´Odontoestomatol 52: 140-144.
- Seppala B, Seppala M, Ainamo J (1993) A longitudinal study on insulin- dependent diabetes mellitus and periodontal disease. J Clin Periodontol 20: 161-165.
- Puig MEF, Reyes OR, Cunill MH, Pacheco NM (2016) Diabetes mellitus y enfermedad periodontal: aspectos fisiopatológicos actuales de su relación. MEDISAN 20: 845.
- Mathur V, Michalowicz BS (1997) Cell-mediated immune system regulation in periodontal diseases. Rev Oral Biol Med 8: 76-89.
- Gurav AN (2013) Advanced glycation end products: a link between periodontitis and diabetes mellitus? Curr Diabetes Rev 9: 355-361.
- Bakshi D, Kaur G, Singh D, Sahota J, Thakur A, et al. (2018) Estimation of plasma levels of tumor necrosis factor-a, interleukin-4 and 6 in patients with chronic periodontitis and type II diabetes mellitus. J Contemp Dent Pract 19: 166-169.
- Seibold JR, Uitto J, Dorward BB, Prockop DJ (1985) Collagen synthesis and collagenase activity indermal fibroblast from patients with diabetes mellitus and digital sclerosis. J Lab Clin Med 105: 664-667.
- Kaplan R, Mulvihill J, Ramamurthy N, Golub L (1982) Gingival collagen metabolism in human diabetics ( Abst). J Dent Res 61: 275.
- Golub LM, Lee HM, Lehrer G (1983) Minocycline reduces gingival collagenolytic activity during diabtes. J Peridontol Res 18: 516-526.
- Zheng J, Chen S, Albiero MI, Vieira GHA, Wang J, et al. (2018) Diabetes activates periodontal ligament fibroblasts via NF-KB in vivo. J Dent Res 97: 1-9.
- Obradors EM, Devesa AE, Salas EJ, Lopez JL, Viñas M (2018) Benefits of non-surgical periodontal treatment in patients with type 2 diabetes mellitus and chronic periodontitis: A randomized controlled trial. J clin Periondotol 45: 345-353.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi