Case Report, Dent Health Curr Res Vol: 3 Issue: 2
Use of Biosilicate® to Treat Bone Defects due to Periapical Disease: A Case Report
Angela Maria Paiva Magri1, Marcelo Donizetti Chaves1, Liciane Bello2, Murilo C. Crovace2 and Ana Claudia M Rennó1*
1Department of Biosciences, Federal University of São Paulo (UNIFESP), Rua Silva Jardim, 136, Vila Mathias, Santos – SP, 11015-020, Brazil
2Department of Material Engineering, Federal University of São Carlos (UFSCar), Rodovia Washington Luís (SP-310), Km 235, 16015-223, São Carlos – SP, Brazil
*Corresponding Author : Ana Claudia Muniz Rennó
Department of Biosciences, Federal University of São Paulo Rua Silva Jardim, 136, Vila Mathias, Santos – SP, 11015-020, Brazil
Tel: +55 13 32218058
Fax: +55 13 32232592
E-mail: a.renno@unifesp.br
Received: July 12, 2016 Accepted: June 19, 2017 Published: July 12, 2017
Citation: Chaves MD, Bello L, Magri AMP, Crovace MC, Rennó ACM (2017) Use of Biosilicate® to Treat Bone Defects due to Periapical Disease: A Case Report. Dent Health Curr Res 3:2. doi: 10.4172/2470-0886.1000127
Abstract
Periapical inflammatory lesion is one of the most prevalent diseases in general dental practices. Many consequences are related to this disease including the appearance of bone defects. In this context, treatments able of stimulating bone tissue have been developed, including osteogenic biomaterials. This pilot case study aimed to evaluate the effects of Biosilicate® in the process of healing in bone defects due to periapical disease. A 53-year-old woman, with recurrent episodes of acute exacerbation of an inflammatory chronic process in the apex of the maxillary left lateral incisor (MLLI), presenting endodontic retreatment and intracanal pin, was studied. Biosilicate® was implanted in the area of the defect and the treatment was analyzed by radiographic examinations (immediately post-surgery, and then 1, 3 and 6 months afterwards). Postoperative pain and swelling were negligible and soft tissue healing was very fast. A higher intensity of pixels in the radiographic examination was observed in the region of the injury after surgery indicating that the material remained in the defect. One month after surgery, the opacity decreased compared to the baseline evaluation, followed by a constant increase 3 and 6 months after surgery. Biosilicate® improved the bone regenerative process, reduced postoperative symptoms and stimulated the deposition of newly formed bone in the area of the bone defect.
Keywords: Periodontal disease; Biomaterials; Glass-ceramics; Biosilicate®; Bone repair
Introduction
Periapical inflammatory lesion is one of the most prevalent diseases in general dental practices [1]. It is the local response of bone around the apex of a tooth that develops after the necrosis of the pulp tissue or extensive periodontal disease [2]. The periapical disease lesion can form a dentoalveolar abscess (acute inflammation) and an apical periodontal cyst apical granuloma (chronic inflammation) [3]. In this context, the development of effective treatments for periapical disease-related problems, such as endondotic and paraendodontic treatments, is of great interest [4].
Also, guided tissue regeneration has been used to stimulate bone healing in periapical diseases, including the use of autogenous and autologous bone [5]. Furthermore, synthetic materials substitutes have been developed as a promising treatment [6].
Engineered bone substitutes are attractive due to their availability and adequate biological and handling properties [7-9]. Materials such calcium phosphate cements, hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP) have been widely explored as alternative synthetic grafts for bone substitution in various types of bone surgery [10].
Additionally, studies have demonstrated the osteogenic properties of the bioactive glasses (BGs) [8]. BGs are a group of synthetic, silicabased bioactive materials with the unique ability to bond to living bone by forming a biologically active bone-like apatite layer on their surface [8]. This layer acts as a template for calcium phosphate precipitation and directs new bone formation [8]. In addition, it has been suggested that BGs attract and stimulate osteoprogenitor cells, which differentiate into matrix-producing osteoblasts, subsequently increasing the rate of bone formation and bone ingrowth into BGbased granular material [11,12]. However, the greatest disadvantages of are their reduced low mechanical strength and fracture toughness [13].
In order to overcome these limitations, our research group developed a fully crystallized glass-ceramic of the Na2O-CaO-SiO2-P2O5 system, with additions of Li2O and K2O, with improved mechanical properties. Many studies have demonstrated the biocompatibility, lack of genotoxicity [14] and osteogenic properties of this material [15-17].
Many experimental studies showed that Biosilicate® produced an acceleration of bone healing in tibial bone defects in healthy and osteoporotic rats [15,16,18].
Also, the efficiency of Biosilicate® in treating dentine hypersensitivity (DH) was demonstrated by Tirapelli et al. [19]. In another study, Martins et al. [20] observed that Biosilicate® exhibits a wide spectrum of antimicrobial properties.
There is a growing interest in the development of materials with improved biological properties to be used as filler materials for bone defects caused by periapical diseases. In view of this, it was hypothesized that Biosilicate® implantation would constitute a therapeutic approach, with high bioactivity rate and ability to accelerate tissue metabolism. The encouraging data from the previous experimental and clinical works formed the basis for the current case report study, which aimed to add knowledge to the existing literature about the effects of Biosilicate® in the treatment of bone defects. A literature survey reveals that there is no documentation of investigations about the effects of this material in the management of large inflammatory periapical lesions.
Case Report
The present study was performed within the guidelines of the World Medical Association Helsinki Declaration of 1975 for biomedical research involving human subjects, as revised in 2000. The present work was approved by the Ethics Committee of the Federal University of São Paulo (number 297022/2012).
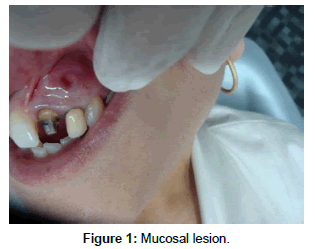
A 53-year-old woman, presenting recurrent episodes of acute exacerbation of an inflammatory chronic process in the apex of the maxillary left lateral incisor (MLLI), with endodontic retreatment and intracanal pin was studied. Intraoral clinical examination revealed painless erythematous swelling of the oral mucosa, with bleeding points and a firm consistency (Figure 1). Tooth decay and periodontal pockets were absent.
In the anamneses, the patient reported the following clinical story: bariatric surgery, type 2 diabetes mellitus, breast cancer triple negative osteopenia and treatment with Risendronate Sodium 35 mg (Norwich Pharmaceuticals Inc., USA), for 3 years. Moreover, the patient informed the presence of lesion persisting after endodontic treatment. The presumptive diagnosis was periapical granuloma.
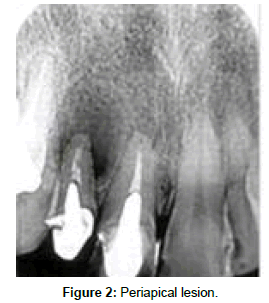
After medical history and clinical examination, periapical and panoramic radiographs were performed and showed a radiolucent periapical area in tooth 22, measuring approximately 10 mm x 6 mm (Figure 2).
Surgical Procedure
Written information about the surgical procedure and the necessary follow-up care was provided to the patient. The treatment protocol, including associated risks was discussed with the patient who then signed a consent form.
The surgical procedure was performed in two phases after the endodontic treatment: removal of the lesion area with subsequent curettage and insertion of the biomaterial in the injured area. Preoperatively, the patient received antibiotic prophylaxis with Azithromycin 500 mg (Novartis, São Paulo, Brazil), one hour before surgery and rinsed her mouth with an antiseptic mouthwash (chlorhexidinedigluconate 0.2% Enila, Rio de Janeiro, Brazil) to reduce the risk of contamination of the surgical field [21]. Using a standardized surgical protocol, the treatment was provided under local anesthesia with mepivacaine 2% with epinephrine (1:100.000 - DLA Pharmaceutical Ltda, São Paulo, Brazil). A submarginal scalloped rectangular (Ochsenbein-Luebke) was the technique used to access the lesion area. The lesion was removed with sharp bone curettes and angled periodontal curettes. The curetted tissue was placed in 10% formalin solution for histologic diagnosis.
Subsequently, bone lesion area was filled with the biomaterial. Biosilicate® (fully-crystallized bioactive glass-ceramic of the quaternary Na2O-CaO-SiO2-P2O5 system) was provided by Vitreous Materials Laboratory (LaMaV), Department of Materials Engineering, Federal University of São Carlos, São Carlos, São Paulo, Brazil [21]. High purity silica, plus reagent grade calcium carbonate, sodium carbonate, and sodium phosphate were used to obtain Biosilicate®. Briefly, the chemicals were weighed and then mixed for 30 minutes in a polyethylene bottle. Premixed batches were melted in platinum crucible at a temperature range of 1250 to 1380°C for 3 hours in an electric furnace (Rapid Temp 1710 BL, CM Furnaces Inc., Bloomfield, NJ, USA). Samples were cast into a 10 mm x 30 mm cylindrical graphite mold and annealed at 460°C for 5 hours. To obtain the fully-crystallized Biosilicate®, Biosilicate® parent glass cylinders underwent cycles of thermal treatment to promote their crystallization. The first thermal cycle was performed at a relatively low temperature, just above the glass transition temperature, to promote volumetric nucleation of crystals. Afterwards, the nucleated samples were submitted to further treatment at about 100oC above the nucleation temperatures. The detailed compositions and thermal treatment schedules to obtain the Biosilicate® are described in the patent WO 2004/074199 [21]. Biosilicate® cylinders were crushed and the powders sieved to select particles in the 180-212 μm range. These were used to fill bone defect in this study.
For implantation, Biosilicate® was manually mixed in a sterile dappen flask with sterile isotonic solution containing 0.9% NaCl (Sanobiol, Minas Gerais, Brazil) to form a consistent paste. Subsequently, the defect area was completely filled with the Biosilicate® clots. After this step, the mucoperiosteal flap was carefully repositioned and sutured with silk thread 3.0 (Ethicon Johnson, São Paulo, São Paulo, Brazil).
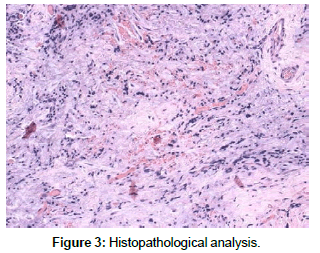
Histopathological analysis revealed extravascular mononuclear cells (chronic inflammation), rich in blood vessels, confirming the presence of periapical granuloma (Figure 3). In addition, the patient reported no edema or pain at all in the first postoperative week, and the soft tissue healing was satisfactory.
Follow Up
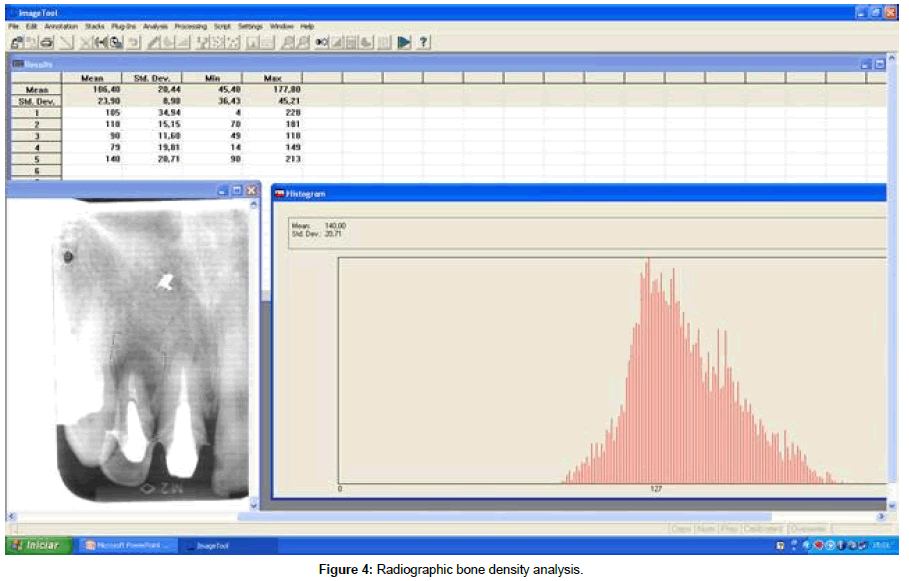
To follow up, periapical radiographs were taken at each scheduled clinical appointment (1, 3 and 6 months), using a long cone paralleling technique. At each clinical appointment, healing was evaluated according to conventional radiography, clinical criteria and radiographic examination of the bone density (Figure 4). Bone density was measured in pixels on a scale ranging from zero (black) to 256 (white) (pixels of 8-bit, 256 gray levels) using the histogram tool program UTHSCSA Image Tool, 3.0 (University of Texas Health Sciences Center, USA).
Clinically, patient recovery was considered successful, as evidenced by the complete restoration of the normal aspect of the mucosa.
Table 1 shows the values of the bone density at the site of the defect during the experimental periods. Immediately after surgery, the value was 118 pixels. Interestingly, one month later, the value of the intensity had decreased to 90 pixels. However, at 3 and 6 months, an increase of the pixel intensity was observed (97 and 140 pixels, respectively), which suggests the installation of the process of repair with neoformation based on gray scale (Table 1).
| Experimental periods | Mean | SD |
|---|---|---|
| Initial lesion Postoperative 1 month 3 month 6 month |
105 118 90 97 140 |
34,94 15,15 11,60 15,33 20,71 |
Table 1: Means and SD of the number pixels.
Discussion
The purpose of the present study was to evaluate and to report the effects of Biosilicate® implantation in a 53-year-old patient, with a large periapical lesion. It was hypothesized that this glass-ceramic would have a positive effect on the formation of bone at the site of the defect. The main findings, demonstrated by the radiographic evaluation, showed that the material remained in the defect area after surgery. Notably, a decrease in the radiographic opacity was observed in the first experimental set point (1 month post-surgey) compared to the baseline values, followed by an increase in density 3 and 6 months after the surgery.
The use of bioactive material is a promising clinical approach to treat bone defects related to periapical disease, especially due to their high bioactivity rate [2,8,11]. In a clinical study of bone defects, Satyanarayana et al. [22] demonstrated a significant improvement in probing depth and bone defect depth due to aggressive periodontitis, when treated with bioglass. Moreover, Pandit et al. [23] showed a reduction in pocket depth and an acceleration of the periodontal osseous defect healing after the BG implantation in the defect [23]. These findings are in line with the results of the current case report, which suggests an increase in the deposition of newly formed bone in the area of the defect studied, as can be observed in the radiographic evaluation (reaching the highest value of opacity 6 months after the procedure). The radiological examination showed that the value of the opacity immediately after the material implantation was higher than the baseline radiographic analysis. This behavior may indicate that the material was successfully implanted and remained at the site of the injury after surgery.
Interestingly, the opacity value decreased one month after the surgery but increased again three and six months post-surgery. It could be suggested that there was a gradually resorption of the material after implantation, followed by its replacement by newly formed bone (demonstrated by the higher values of opacity in the later evaluations).
It is well known that resorption of the bone substitute material (e.g. biodegradation of the material) is required for formation of new bone tissue and tissue in growth in the defect area [24,25]. The radiographic results of the current study indicate that the degradation rate of the material may have substantially influenced the formation of bone, indicated by the higher values of opacity showed in the last period evaluated. This fact might be related to the ion dissolution from the Biosilicate®, which occurs immediately after the material comes into contact with body fluids [8,16].
Upon implantation, Biosilicate® ionic dissolution products of have been shown to beneficially affect osteogenesis by the formation of a silica-rich layer that acts as a template for calcium phosphate precipitation and directs new bone formation [12,13].
Furthermore, it has been reported that Biosilicate® has a stimulatory effect on neovascularisation by stimulating the secretion of angiogenic factors [17], which, together with the osteopromotive properties of Biosilicate® might further influence bone formation.
This case report suggests that Biosilicate® may favorably affect hard tissue healing after the surgical procedure, minimizing patients’ postoperative discomfort. Moreover, the outcome of the current study confirmed our initial hypothesis that Biosilicate® would improve bone metabolism, accelerating the healing process.
Consequently, Biosilicate® might be a promising material to be used as bone graft in compromised conditions. As this study was limited to a single case report and a relatively short-term evaluation of the ability of preset material to provide full control over the complete filling of the defect, information on the longterm performance of the material, in a randomized clinical trial, remains to be provided.
Conclusion
In the patient studied, there was an increase in density in the radiographic examinations of Biosilicate®, which may indicate a stimulation of bone healing and an increase in bone formation in the defect area. Although this study is a case report, our data highlights the potential of Biosilicate® to be used as a promising treatment for bone regeneration applications. Further long-term studies should be carried out, involving a larger number of patients, to provide additional information about the bone regeneration induced by Biosilicate®.
References
- Xia WW, Zhu YQ, Wang XY (2011) Six cases report of differential diagnosis of periapical diseases. Int J Oral Sci 3: 153-159.
- Desai SR, Shinde HH (2012) Correlation of interdental and interradicular bone loss in patients with chronic periodontitis: a clinical and radiographic study. Niger J Clin Pract 15: 125-131.
- Boyapati L, Wang HL (2006) The role of platelet-rich plasma in sinus augmentation: a critical review. Implant Dent 15: 160-170.
- Di Stefano DA, Andreasi Bassi M, Cinci L, Pieri L, Ammirabile G (2012) Treatment of a bone defect consequent to the removal of a periapicalcyst with equine bone and equine membranes: clinical and histological outcome. Minerva Stomatol 61: 477-490.
- Artzi Z, Wasersprung N, Weinreb M, Steigmann M, Prasad HS, et al. (2012) Effect of guided tissue regeneration on newly formed bone and cementum in periapical tissue healing after endodontic surgery: an in vivo study in the cat. J Endod 38: 16316-9.
- Giannoudis PV, Chris Arts JJ, Schmidmaier G, Larsson S (2011) What should be the characteristics of the ideal bone graft substitute? Injury 42 Suppl 2: S1-2.
- Välimäki VV, Yrjans JJ, Vuorio EI, Aro HT (2005) Molecular biological evaluation of bioactive glass microspheres and adjunct bone morphogenetic protein 2 gene transfer in the enhancement of new bone formation. Tissue Eng 11: 387-394.
- Hench LL (2006) The story of Bioglass. J Mater Sci Mater Med 17: 967-978.
- Dias AG, Lopes MA, Santos JD, Afonso A, Tsuru K, et al. (2006) In vivo performance of biodegradable calcium phosphate glass ceramics using the rabbit model: histological and SEM observation. J Biomater Appl 20: 253-266.
- Link DP, van den Dolder J, van den Beucken JJ, Habraken W, Soede A, B et al. (2009) Evaluation of an orthotopically implanted calcium phosphate cement containing gelatin microparticles.J Biomed Mater Res A 90: 372-379.
- Jones JR (2013) Review of bioactive glass: from Hench to hybrids. Acta Biomater 9: 4457-4486.
- Day RM, Maquet V, Boccaccini AR, Jérôme R, Forbes A (2005) In vitro and in vivo analysis of macroporous biodegradable poly(D,L-lactide-co-glycolide) scaffolds containing bioactive glass. J Biomed Mater Res A 75: 778-787.
- Peitl O, Zanotto ED, Serbena FC, Hench LL (2012) Compositional and microstructural design of highly bioactive P2O5-Na2O-CaO-SiO2 glass-ceramics. Acta Biomater 8: 321-332.
- Kido HW, Oliveira P, Parizotto NA, Crovace MC, Zanotto ED, et al. (2013) Histopathological, cytotoxicity and genotoxicity evaluateion of Biosilicate® glass-ceramic scaffolds. J Biomed Mater Res A 101: 667-673.
- Granito RN, Rennó AC, Ravagnani C, Bossini PS, Mochiuti D, et al. (2011) In vivo biological performance of a novel highly bioactive glass-ceramic (Biosilicate®): A biomechanical and histomorphometric study in rat tibial defects. J Biomed Mater Res B Appl Biomater 97: 139-147.
- Renno AC, Bossini PS, Crovace MC, Rodrigues AC, Zanotto ED, et al. (2013) Characterization and in vivo biological performance of biosilicate. Biomed Res Int 2013: 141427.
- Bossini PS, Rennó AC, Ribeiro DA, Fangel R, Peitl O, et al. (2011) Biosilicate® and low-level laser therapy improve bone repair in osteoporotic rats. J Tissue Eng Regen Med 5: 229-237.
- Oliveira P, Ribeiro DA, Pipi EF, Driusso P, Parizotto NA, et al. (2010) Low level laser therapy does not modulate the outcomes of a highly bioactive glass-ceramic (Biosilicate®) on bone consolidation in rats. J Mater Sci Mater Med 21: 1379-1384.
- Tirapelli C, Panzeri H, Soares RG, Peitl O, Zanotto ED (2010) A novel bioactive glass-ceramic for treating dentin hypersensitivity. Braz Oral Res 24: 381-387.
- Martins CH, Carvalho TC, Souza MG, Ravagnani C, Peitl O, et al. (2011) Assessment of antimicrobial effect of Biosilicate® against anaerobic, microaerophilic and facultative anaerobic microorganisms. J Mater Sci Mater Med 22: 1439-1446.
- Zanotto ED, Ravagnani C, Peitl O, Panzeri H, EH L (2004) Process and compositions for preparing particulate, bioactive or resorbable biosilicates for use in the treatment of oral ailments, WO2004/074199, Fundação Universidade Federal De São Carlos; Universidade De São Paulo, Brazil.
- Satyanarayana KV, Anuradha BR, Srikanth G, Chandra PM, Anupama T, et al. (2012) Clinical evaluation of intrabony defects in localized aggressive periodontitis patients with and without bioglass- an in-vivo study. Kathmandu Univ Med J (KUMJ) 10: 11-15.
- Pandit N, Gupta R, Gupta S (2011) A comparative evaluation of biphasic calcium phosphate material and bioglass in the treatment of periodontal osseous defects: a clinical and radiological study. J Contemp Dent Pract 11: 25-32.
- Ruhé PQ, Hedberg EL, Padron NT, Spauwen PH, Jansen JA, et al. (2005) Biocompatibility and degradation of poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composites. J Biomed Mater Res A 74: 533-544.
- Qi X, Ye J, Wang Y (2008) Improved injectability and in vitro degradation of a calcium phosphate cement containing poly(lactide-co-glycolide) microspheres. Acta Biomater 4: 1837-1845.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi