Case Report, J Genet Disor Genet Rep Vol: 7 Issue: 3
Atypical Hypotonia-Cystinuria a New Case: Genotype-Phenotype, Description
Preka Evgenia1,3*, Ariane Paoloni-Giacobino2, Frédérique Sloan-Béna2, Paloma Parvex3 and Alexandra Wilhelm-Bals3
1Great Ormond Street Hospital, WC1N 3JH London, UK
2Department of Genetic and Laboratory Medicine, Geneva University Hospital, 1211 Geneva 14, Switzerland
3Department of Paediatric Nephrology, Geneva University Hospital for Children, Rue Willy-Donzé 6, 1211 Geneva 14, Switzerland
*Corresponding Author : Evgenia Preka
Departement of Paediatric Nephrology, Geneva University Hospital for Children, Rue Willy-Donzé 6, 1211 Geneva 14, Switzerland
Tel: +0044 20 7762 6725
E-mail: evgenia.preka@gmail.com
Received: October 10, 2018 Accepted: November 08, 2018 Published: November 15, 2018
Citation: Evgenia P, Paoloni-Giacobino A, Sloan-Béna F, Parvex P, Wilhelm-Bals A (2018) Atypical Hypotonia-Cystinuria a New Case: Genotype-Phenotype, Description. J Genet Disor Genet Rep 7:3. doi: 10.4172/2327-5790.1000180
Abstract
“Hypotonia-cystinuria syndrome” (HCS) and “the 2p21 deletion syndrome” are two recessive contiguous gene deletion syndromes associated with cystinuria. The deletions differ in size and number of genes involved. HCS is characterized by hypotonia, failure to thrive, severe growth retardation, growth hormone deficiency, characteristic facial dysmorphy and cystinuria. In addition to mental retardation and respiratory chain complex deficiency, the 2p21 deletion syndrome presents HCS features. In HCS, SLC3A1 and PREPL genes are disrupted, while in the 2p21 deletion syndrome two additional genes (C2orf34 and PM1B) are deleted. Mutations in SLC3A1 are known to cause cystinuria. The extended phenotypes are attributed to PREPL and C2orf34 PM1B deletions. While HCS is described in 19 families, 2p21 syndrome is only in one Bedouin family. An intermediate phenotype, resulting from deletion of SLC3A1, PREPL and C2orf34 has been reported twice and is known as “atypical HCS”. We will describe a new case of “atypical HCS” and its genotype-phenotype correlation. Array-CGH evidenced a homozygote microdeletion in chromosome 2p21 partially including genes SLC3A1 and CAMKMT and complete inclusion of the PREPL gene. Both parents showed the same deletion in the heterozygous state. In contiguous deletion syndromes, characterization of the different deletions is crucial for precise genotype-phenotype correlation and to understand the role of each gene.
Keywords: Atypical hypotonia-cystinuria syndrome; 2p21 microdeletion; Neonatal hypotonia; Homozygous deletion syndrome
Introduction
Despite the fact that the hallmark of hypotonia-cystinuria syndrome (HCS; OMIM 606407) is the presence of cystinuria type I (disruption on the SLC3A1 locus at chromosome 2p21), recessive deletions in contiguous genes in the same chromosome result in variable phenotypes depending on the different size and number of the deleted genes involved. In general, the inherited contiguous gene microdeletion syndromes in a recessive mode are very rare but recent literature brings into view more phenotype-genotype associations trying to explain their etiopathogenic mechanisms.
HCS patients, only SLC3A1 and PREPL genes are disrupted, whereas in the 2p21 deletion syndrome, two additional genes (C2orf34 and PM1B) are deleted [1]. The HCS syndrome is characterized by generalized hypotonia at birth while failure to thrive, and severe growth retardation due to growth hormone deficiency, characteristic facial dysmorphy and cystinuria type 1 appear later in childhood. As mutations in SLC3A1 cause isolated cystinuria type 1, the extended phenotype can be attributed to PREPL [2].
To date, HCS has been described in 19 families with different deletions [3] having in common that both alleles of SLC3A1 and PREPL were deleted. The 2p21 deletion syndrome has been described in a small Bedouin clan, with a deletion of 179 kb disrupting SLC3A1, PREPL, PPM1B and C2orf34 [4]. The 2p21 deletion syndrome presents features of hypotonia-cystinuria syndrome in addition to mental retardation, elevated serum lactate concentration and respiratory chain complex deficiency in some patients.
An intermediate phenotype to HCS and 2p21 deletion syndrome resulting from the deletion of SLC3A1, PREPL and C2orf34 has been reported twice in two siblings, and termed “atypical HCS” [1,5]. Bartholdi et al, described another case of two siblings carrying a microdeletion in PREPL and C2orf34 (CAMKMT) genes, but not the SLC3A1 gene which had a recognizable phenotype to atypical HCS but without cystinuria [6].
Here we report a new case of “atypical HCS” 2p21 microdeletion, characterized by array-CGH, deleting SLC3A1, PREPL and C2orf34 and the resulting phenotype of the patient, trying to put further insight in the phenotype-genotype correlations of those microdeletion syndromes. The more precise the phenotypic characterization is, the more targeted the genotype testing and the genetic counseling for the affected families.
Case Report
We report the case of a 4 years old girl referred to our center for a second opinion. The girl is the third child of a healthy, consanguineous Libyan family. The parents are second-degree cousins with an unremarkable background and their two other children, are healthy. The patient was born at full term by cesarean section for failure of induction and her birth weight was 2.6 kg. At birth, the patient was described with severe central hypotonia, feeding difficulties and failure to thrive. A Brain MRI was performed and showed no abnormalities. During her first months of life, she was handled with nasogastric tube feeding and she managed to follow her percentile (below 5th) constantly.
At 3 years, the abdominal echography revealed bilateral nephrocalcinosis and the patient started presenting multiple urinary lithiasis and urinary tract infections. Urine testing confirmed cystinuria with 24hour urine showing high cystine/creatinine ratio (696 μmol/L/24h). Otherwise the biochemical investigations were normal (normal kidney function, normal plasma electrolytes) except for slightly increased urinary oxalate (0.4 mmol/24h). Hyperhydratation and alkalinisation of the urine with bicarbonate supplementation were started with poor results due to difficulty of administration.
At 8 months, the girl weighed 5.2 kg (still p<5th), with a head circumference of 41.5 cm (5th percentile). She was still very floppy and was slow to reach and grasp objects according to her expected agerelated milestones. She started to walk at 2 years old with intensive physiotherapeutic support and started to say a few words at the age of 3.
As the patient was developing severe growth and developmental delay, and the etiology was unclear, the girl was referred for a second opinion to our center.
On admission to our unit, at the age of 4 years old, she weighed 9.6 kg (<<3rd centile) and her height was 89 cm (<<3rd centile). When walking, which was rare, she frequently fell and was not able to construct sentences due to incomprehensible hyper nasal speech. She was described as timorous and shy with episodes of weakness and fatigue.
The patient presented craniofacial dysmorphia including pale skin as compared to the rest of her family, a long face with mild bitemporal narrowing and frontal bossing, a turned up nose, tented upper lip, protruding ears and slight bilateral palpebral ptosis.
The laboratory results revealed a positive Brand test, and urine amino acid chromatography confirmed the diagnosis of classical cystinuria with increased cystine, lysine, arginine and ornithine. Renal ultrasound showed multiple massive corral form obstructive calculus. Renal function was normal. Endocrinology-wise the investigations reveled low IGF-1 levels, glucagon testing could not be performed. Thyroid function was normal. Venous acid-base status and lactate were normal. Because of the suggestive phenotype, we decided to explore a possible genomic imbalance by array-CGH on peripheral blood DNA from the patient.
Oligonucleotide array-CGH was performed on DNAs extracted from lymphocytes with an 180,000-oligonucleotide microarray (Sure Print G3 Human 4x180k CGH Microarray Kit, Agilent Technologies, Santa Clara, CA) according to the manufacturer’s protocol. A commercial Control DNA was used (Promega). The slides were scanned on an Agilent DNA Microarray Scanner and images were extracted with Feature Extraction software (9.5.3.1). Data analysis was carried out with the DNA Analytics software (4.0.85). The following parameters were used for interpretation: ADM-2: 5.6, z-score: 2, window: 0.2 Mb, cutoff: 0.25 and scatter-plot: 0.25. At least three contiguous oligonucleotides presenting an abnormal log ratio were considered necessary to qualify for a CNV. Results are given in hg19 genome version. To qualify as a heterozygous deletion the log2ratio Cy5/Cy3 is expected to be -0.8 to 1.
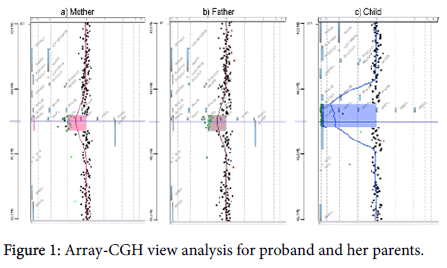
The array-CGH analysis evidenced an identical homozygote microdeletion in chromosome 2p21 (chr2:44507915-44757213pb) with a minimum size of 250 kb partially including the genes SLC3A1 (exons 3-6), and KAMCAT (exons 1-2) and the entire gene PREPL (Figure 1). We also performed the array-CGH analysis on the parents of the patient, which showed a heterozygous microdeletion in each of them. Therefore the patient has inherited identical deletions from both parents (Figure 1 (a,b)).
Array-CGH data plot for chromosome 2 in p21 region showing the heterozygous deletion in the mother (a), the father (b) and the homozygous deletion in the proband (c). The shaded blue regions indicate the deletions, which reflect deviation from the log2 ratio of zero. The homozygous deletion is indicated by an average probe value of -4 and the heterozygous deletion is shown by an average probe value of -1.The gene names are indicated in grey on the patient‘s image.
Discussion
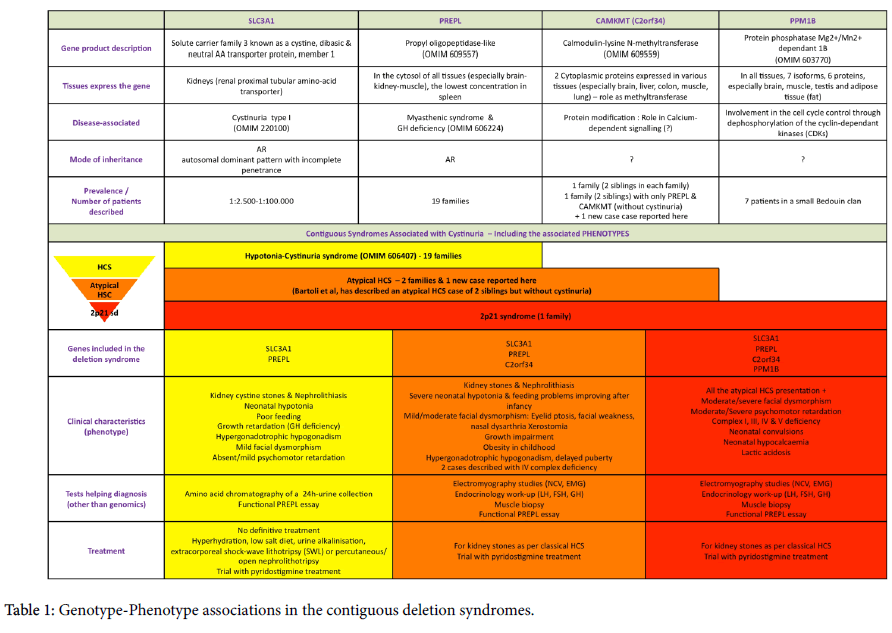
The recent advances in medical technology give more insight into the four genes involved in the contiguous deletion syndromes at the 2p21 locus (SLC3A1, PREPL, CAMKMT and PPM1B) associated with cystinuria. The main phenotype characteristics of the deletion syndromes, their pathomechanisms, epidemiology and actual possibilities in diagnosis and treatment are summarized in Table 1.
In our novel case, the identification of the three different deletions affecting the SLC3A1, CAMKMT (C2orf34) and the entire PREPL confirmed our clinical suspicion of atypical HCS in this patient [1]. Until now, only four patients (two pairs of siblings) have been reported in the literature with atypical hypotonia-cystinuria syndrome and the entire phenotype-genotype associations in most of the microdeletion syndromes is still not entirely defined.
The more common and least severe among the homozygous 2p21 deletion syndromes, the hypotonia cystinuria syndrome, is characterized by neonatal hypotonia, growth hormone deficiency, minor facial dysmorphism, failure to thrive in the early years followed by hyperphagia and obesity in late childhood, and classical cystinuria. The molecular defect of SLC3A1 function is known to cause the isolated cystinuria type I, and subsequently all the other features were attributed to PREPL deficiency [7] (Table 1).
The gold standard for the early diagnosis of cystinuria remains the quantitative cystine excretion by amino-acid chromatography regardless of the underlying gene defect [8]. A novel screening test for PRELP deficiency has been developed recently. Using both screening tools, the clinician could be guided promptly when there is a suspicion of HCS (or another contiguous deletion syndrome), when genetics are pending or are not feasible. The new functional blood assay for identifying PREPL deficiency was developed by Regal et al by using stimulated lymphocytes. The new assay is able to distinguish between patients suffering from PREPL deficiency (abnormal result) and patients with Prader-Willy syndrome or other final neuromuscular diseases that showed normal results [5].
Besides to the specific screening test for PREPL deficiency, Regal et al, described the first patient with isolated Propyl-Oligopeptidase like (PREPL) deficiency who presented having myasthenic symptoms since birth and a positive edrophonium test with pre- and postsynaptic features as well as growth hormone deficiency [9]. PREPL was found to encode a cytoplasmic serine hydrolase structurally belonging to an oligopeptidase family, which, being highly expressed in brain, muscle and kidney [10], was categorized as a synaptic protein. Additionally, Lone et al [11] found that Prepl-null mice were significantly smaller than wildtype littermates and showed neonatal hypotonia. The findings suggested a role for PREPL in the developing musculoskeletal system.
Moreover, Regal et al, described a neuromuscular junction defect and a transient improvement when pyridostigmine was administered to a patient with isolated PREPL deficiency and to another patient with typical HCS (defects on both SLC3A1 and PREPL genes) [9]. In his recent cohort with 5 patients, characterized by isolated PREPL deficiency [5], all the subjects presented severe neonatal hypotonia, nasal dysarthria and/or neonatal feeding problems. However, ptosis and facial weakness were not constant findings in those patients and as such, they were attributed to orthosympathetic involvement. Interestingly his patients showed a variable cognitive function, from mild to normal intelligence.
Interestingly, in the DECIPHER database, one case of homozygous deletions including part of both genes SLC3A and PREPL (Patient ID: 286346) [12] is described in a patient with poor sucking reflex, cystinuria and hyperreflexia, but without any evidence of hypotonia. However, the age that the hypotonia is presented in each subject and the degree of that hypotonia is variable in the literature. In any case, as the severe neonatal hypotonia in PREPL deficiency might improve with age, and there might be a significant role of pyridostigmine treatment in the clinical outcome, early diagnosis and prenatal counseling are extremely important.
Until recently, the role of calmodulin lysine transferase (CaM KMT or C2orf34) was not clear in human patients. Parvari et al, developed a knock out mouse with selective deletion of the C2orf34 (CAMKMT) gene and found that impairment of complexes I, and IV and less significantly III, of the mitochondrial respiratory chain were more pronounced in the brain than in the muscle [13]. Consequently, this translational research mouse model gives more insight into the role of CAMKT gene in the 2p21 deletion syndrome, as it finally affects the motor learning, the complex coordination and the learning of aversive stimuli. Overall, their mouse model underlines the importance of the methylated CaM in growth, muscle strength, somatosensory development and brain function.
In human patients, the accumulation of hypomethylated CaM had already been described to contribute to the mental retardation and mitochondrial defect in 2p21 deletion syndrome [14]. Obviously, the loss of this additional gene (C2orf34) adds to the complex and severe phenotype in atypical HCS.
Additional deletion of the Protein Phosphatase 1B, Magnesium- Dependent, Beta Isoform (PPM1B) gene causes the 2p21 syndrome (previously described as 2p16) [15] known for its severity as many effected children have been described to present from severe neonatal seizures to severe mitochondrial dysfunction, severe psychomotor retardation and finally death [4]. The knockout mouse model of Sasaki et al, delineates the fundamental role of PPM1B in the gametogenesis, fertilization and early stages of embryonic development, with the model embryos being lethal between the two-cell and eight-cell stages [16]. Additionally, Chen et al, demonstrated that PPM1B protects mice from TNF-induced SIRS through dephosphorylating Rip3 and their data suggest that the absence of PPM1B regulates necroptosis in vivo and in vitro [17].
Common clinical features between HCS and homozygous 2p21 syndrome include: cystinuria type I, neonatal hypotonia with poor feeding and growth retardation (Table 1). Although in all syndromes growth retardation is present, only in HCS is there a GH deficiency where patients respond to treatment with GH therapy. The homozygous deletion of PREPL has been defined and associated mostly with growth retardation with GH deficiency and hypotonia [7,9]. Moreover, studies in PREPL -/- mice have shown diminished growth and neonatal hypotonia compared to their wild type PREPL +/+ counterparts [11].
However, in a recent cohort of 10 patients with confirmed PREPL deletion, only 4 patients present GH deficiency (three with isolated PREPL deficiency and one with HCS) at different ages from 20 months to 11 years [5]. Consequently, the role of PREPL deletion in GH hormone function remains to be explained, especially when this effect is not regularly present on humans with isolated PREPL deficiency. On the contrary, GH hormone deficiency has not been demonstrated in any atypical HCS and only in 1 patient with 2p21 syndrome [6]. In 2p21 syndrome, the mechanism of growth retardation is still unknown, patients present more prominent facial dysmorphic characteristics, neonatal seizures, hypocalcemia, lactic acidosis, decreased activity of the respiratory chain complexes I, III, IV, V and moderate to severe psychomotor retardation (Table 1) [16].
In addition to the foregoing and considering the frequent presence of hypergonadotrophic hypogonadism in HCS and in isolated PREPL deficiency, this clinical finding could be attributed to the PREPL gene defect [5].
Without doubt, the 2p21 syndrome is the most severe among the contiguous gene deletion syndromes including a moderate to severe psychomotor retardation and a decrease in activity of the respiratory chain complexes I, III and IV [18]. We suggest that the possible role of C2orf34 and PPM1B form the difference in the phenotypes of atypical HCS and the 2p21 syndrome. Consequently and regarding the literature until now, C2orf34 or PPM1B deficiency should be suspected in cases of mitochondrial dysfunction or respiratory chain disorders.
Our new case of atypical HCS adds more refinement to the genotype-phenotype associations in contiguous deletion syndromes. Chabrol et al described the first case of atypical HCS, in which two siblings presented a mild/moderate mental retardation and one of them presented a respiratory chain complex IV deficiency [1]. The two siblings were diagnosed with breakpoints in the SLC3A1, PREPL and C2orf34 genes. Unexpectedly the nerve conductive velocity (NCV) tests for both siblings were normal, but their electromyography (EMG) study showed little myotonic activity and chronic denervation. The two siblings had muscle biopsies that demonstrated hypotrophy of fibers III, as well as nuclear inclusions. Due to the young age of our patient it was, for practical reasons, impossible to do NCV, EMG or muscle biopsy without sedating her for practical reasons, so we avoided this test, as the genetic results directly confirmed our clinical hypothesis.
Intriguingly, Bartholdi et al, described two siblings encompassing the PREPL and C2orf34 genes deletion, without disrupting the SLC3A1 gene [6]. Their patients, characterized as atypical HCS cases presented IUGR, severe hypotonia with feeding problems and lactic acidosis in the neonatal period, facial distinct features, growth impairment (without GH deficiency), hypernasal speech, mild intellectual disability and genitalia problems. After the elucidation of PREPL isolated deficiency [8] the hypotonia and the myasthenic symtoms can be attributed to PREPL deletion. Remarkably the GH test was normal in both patients, which means that their failure to thrive might be attributed to C2orf34 deletion. Mice homozygous for knockout alleles in C2orf34 are known to exhibit reduced body weight, reduced muscle strength and altered somatosensory development and brain function [13].
Patients presenting slow growth and muscle weakness that could result from a mitochondrial impairment and mental retardation should be considered for sequence analysis of the CaM KMT gene. Moreover, patients presenting mild hypotonia or symptoms similar to Prader-Willy syndrome but with negative genetics might benefit from the PREPL blood assay test and its genetic analysis.
The field of clinical genetics has progressed considerably with the development of array CGH. This sophisticated tool allows detection of smaller scale genetic changes and chromosomal imbalances such as microdeletions, and microduplications. Although it has some disadvantages [for instance, not being able to detect aneu-/polyploidy reliably, it’s uncertain clinical significance for multiple copy number variations and the fact that low level mosaicism can be present (up to 8%)], array-CGH use in pre- and postnatal setting is growing as it can detect more subtle changes in genetic material and can be used to evaluate from the entire genome to targeted areas of interest [19,20]. Indeed, it can permit testing at risk pregnancies in a family such as the one we have reported, i.e. each parent carrying a microdeletion in heterozygous state. In order to anticipate the ethical issues, therapeutic or preventive measures related to this genetic counseling should be reported ahead and clinicians should inform the patients for the risk of incidental findings [21].
Conclusion
In summary, a new patient is reported to be carrying a homozygous deletion in the 2p21 region, including three genes: SLC3A1, PREPL and C2orf34. In the first two families described with aHCS, both of which had two children affected, the same phenotype has been reported.
Our case, in association with the recent literature, further elucidates the complex genotype-phenotype correlations of the contiguous deletion syndromes associated with cystinuria. The phenotypic refinement reported in this paper together with the new screening tool for PREPL deficiency and the progress of medical genetics with the array CGH in pre- and post-natal settings make nowadays a reliable diagnosis of contiguous deletion syndromes feasible.
Acknowledgments
The authors would like to express their gratitude to the family and the local hospitals in Libya for the information provided.
References
- Chabrol B, Martens K, Meulemans S, Cano A, Jaeken J, et al. (2009) Deletion of C2orf34, PREPL and SLC3A1 causes atypical hypotonia-cystinuria syndrome. J Med Genet 45: 21686663.
- Martens K, Heulens I, Meulemans S, Zaffanello M, Tilstra D, et al. (2007) Global distribution of the most prevalent deletions causing hypotonia-cystinuria syndrome. Eur J Hum Genet 15: 1029-1033.
- Regal L, Aydin HI, Dieltjens AM, Van Esch H, Francois I, et al. (2012) Two novel deletions in hypotonia-cystinuria syndrome. Mol Genet Metab 107: 614-616.
- Parvari R, Gonene Y, Aishafee I, Buriakovsky S, Regev K, et al. (2005) The 2p21 deletion syndrome: Characterization of the transcription content. Genomics 86: 195-211.
- Regal L, Martensson E, Maystadt I, Voermans N, Lederer D, et al. (2018) PREPL deficiency: Delineation of the phenotype and development of a functional blood assay. Genet Med 20: 109-118.
- Bartholdi D, Asadollahi A, Oneda B, Schmitt-Mechelke T, Tonella P, et al. (2013) Further delineation of genotype-phenotype correlation in homozygous 2p21 deletion syndromes: First description of patients without cystinuria. Am J Med Genet A 161A: 1853-1859.
- Jaeken J, Martens K, Francois I, Eyskens F, Lecointre C, et al. (2006) Deletion of PREPL, a gene encoding a putative serine oligopeptidase, in patients with hypotonia-cystinuria syndrome. Am J Hum Genet 78: 38-51.
- Knoll T, Zollner A, Wendt-Nordahl G, Michel MS, Alken P (2005) Cystinuria in childhood and adolescence: Recommendations for diagnosis, treatment, and follow-up. Pediatr Nephrol 20: 19-24.
- Regal L, Shen XM, Selcen D, Verhille C, Meulemans S, et al. (2014) PREPL deficiency with or without cystinuria causes a novel myasthenic syndrome. Neurology 82: 1254-1260.
- Radhakrishnan K, Baltes J, Creemers JW, Schu P (2013) Trans-Golgi network morphology and sorting is regulated by propyl-oligopeptidase-like protein PREPL and the AP-1 complex subunit μ1A. J Cell Sci 126: 1155-1163.
- Lone AM, Leidl M, McFedries AK, Horner JW, Creemers J, et al. (2014) Deletion of PREPL causes growth impairment and hypotonia in mice. PLoS One 9: e89160.
- DECIPHER (2018) DatabasE of genomic variation and Phenotype in Humans using Ensemble Resources. Wellcome Sanger Institute. Accessed on: 15 October 2018.
- Haziza S, Magnani R, Lan D, Keinan O, Saada A, et al. (2015) Calmodulin Methyltransferase is required for growth, muschle strenth, somatosensory development and brain function. PLoS Genet 11: e1005388.
- Magen S, Magnani R, Haziza S, Hershkovitz E, Houtz R, et al. (2012) Human calmodulin methyltransferase: Expression, activity on calmodulin, and Hsp90 dependence. PLoS One 7: e52425.
- Parvari R, Brodyansky I, Elpeleg O, Moses E, Landau D, et al. (2001) A recessive contiguous gene deletion of chromosome 2p16 associated with Cystinuria and a mitochondrial disease. Am J Hum Genet 69: 869-875.
- Sasaki M, Ohnishi M, Tashiro F, Niwa H, Suzuki A, et al. (2007) Disruption of the mouse protein Ser/Thr phosphatase 2Cbeta gene leads to early pre-implantation lethality. Mech Dev 124: 489-499.
- Chen W, Wu J, Li L, Zhang Z, Ren J, et al. (2015) Ppm1b negatively regulates necroptosis through dephosphorylating Rip3. Nat Cell Biol 17: 434-444.
- Martens K, Jaeken J, Matthijs G, Creemers JW (2008) Multi-system disorder syndromes associated with Cystinuria type I. Curr Mol Med 8: 544-550.
- Evangelidou P, Sismani C, Ioannides M, Christodoulou C, Koumbaris G, et al. (2010) Clinical application of whole-genome array CGH during prenatal diagnosis: Study of 25 selected pregnancies with abnormal ultrasound findings or apparently balanced structural aberrations. Mol Cytogenet 3: 24.
- Sang-Jin P, Eun Hye J, Ran-Suk R, Hyun Woong K, Jung-Min K, et al. (2011) Clinical implementation of whole-genome array CGH as a first-tier test in 5080 pre and postnatal cases. Mol Cytogenet 4: 12.
- Lefebvre M, Sanlaville D, Marle N, Thauvin-Robinet C, Gautier E, et al. (2016) Genetic counseling difficulties and ethical implications of incidental findings from array-CGH: a 7-year national survey. Clin Genet 89: 630-635.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi