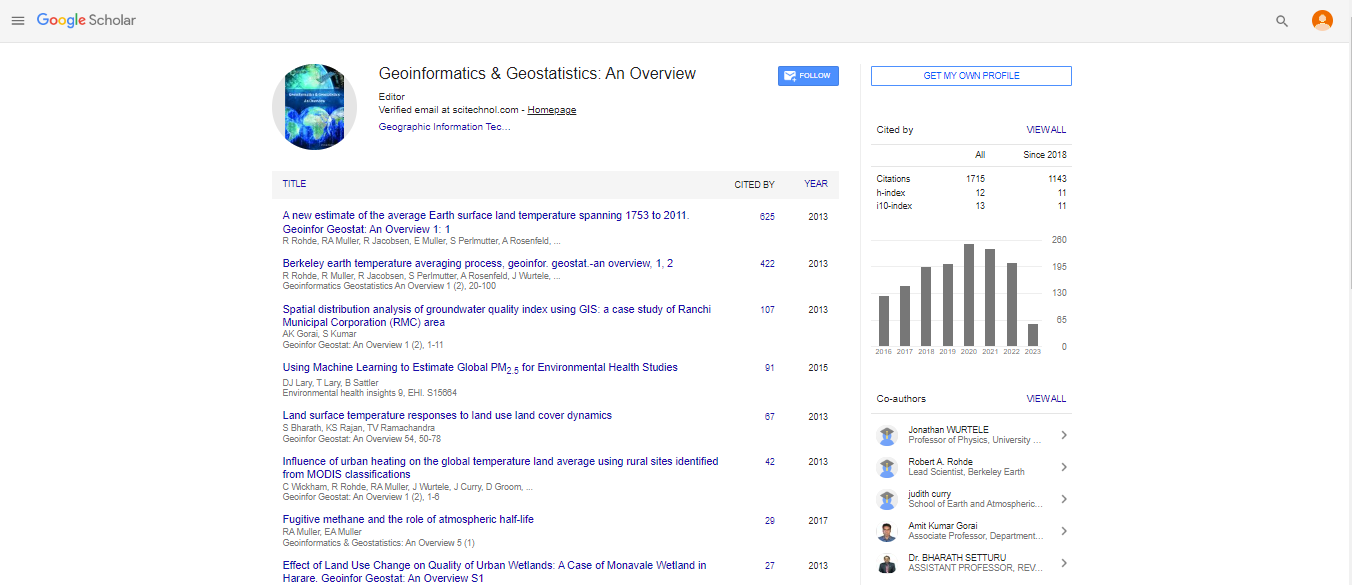
Short Communication, Geoinfor Geostat An Overview Vol: 4 Issue: 2
Ecology and Biogeography, Future Perspectives: Example Marine Parasites
| Rohde K* | |
| Professor emeritus, Zoology, School of Environmental and Rural Science, University of New England, Armidale, Australia | |
| Corresponding author: Klaus Rohde Klaus Rohde, School of Environmental and Rural Science, University of New England, Armidale, Australia Tel: +61 2 6772 6069 E-mail: krohde@une.edu.au |
|
| Received: February 22, 2016 Accepted: April 12, 2016 Published: April 19,2016 | |
| Citation: Rohde K (2016) Ecology and Biogeography, Future Perspectives: Example Marine Parasites. Geoinfor Geostat: An Overview 4:2. doi:10.4172/2327-4581.1000140 |
Abstract
Ecology and Biogeography, Future Perspectives: Example Marine Parasites
This article draws attention to some perspective that must be considered in future research in biogeography and ecology. Both disciplines are closely connected: biogeography, the study of biodiversity and its patterns in space and time, depends on ecology, the study of interactions between organisms and their environment, for elucidating reasons for the patterns.
Keywords: Biodiversity; Marine parasites; Biogeographical patterns; Vacant niches; Agent based models; Effective evolutionary time
Keywords |
| Biodiversity; Marine parasites; Biogeographical patterns; Vacant niches; Agent based models; Effective evolutionary time |
Introduction |
| This article draws attention to some perspective that must be considered in future research in biogeography and ecology. Both disciplines are closely connected [1]: biogeography, the study of biodiversity and its patterns in space and time, depends on ecology, the study of interactions between organisms and their environment, for elucidating reasons for the patterns. Aspects considered are: |
| 1) What do we know about biodiversity? |
| 2) Biogeographical patterns |
| What do we know about biodiversity? |
| Aguiar et al. [2] discussed global speciation and diversity, and Appletans et al. [3] the magnitude of marine species diversity. The latter authors conclude that “between one-third and two-thirds of marine species may be undescribed” and that” If the current trends continue, most species will be described this century”. Recent reviews of parasite diversity in general are given by the various contributors in Morand and Krasnov [4] and Morand et al. [5]. Detailed discussions of marine parasite diversity can be found in Rohde [6,7] and Leung et al. [8]. These reviews show that even hosts (and particularly invertebrate hosts) of parasites are incompletely know, i.e., many species have not been described. Leung et al. [8] gave estimates of known species and those estimated to exist, of some important groups of marine invertebrates using the Catalog of Life database (www.species2000org) and the World Registry of Marine Species (www.marinespecies.org). Some examples are: Cnidaria 11 433 species known, 40 318 estimated to exist; Echinodermata: 7286 vs. 19040; Mollusca: 48 648 vs. 169 840;Crustacea: 66 250 vs. 130 855. Parasites are far less known. Much work on parasites remains to be done even for well-known marine host animals such as seabirds. Hoberg [9] reports that more than 700 species of digenean trematodes, eucestodes, nematodes and acanthocephalans have been described from about 165 marine bird species. 50% of all bird species have never been examined. Protistan parasites of marine invertebrates and vertebrates are less well known, and studies have been mainly restricted to economically important species. For example, there are thousands of marine fish species in Australia, but probably less than 5% of them have been examined for protistan parasites [7].Species richness on the Great Barrier Reef, the largest complex of coral reefs on earth, has been extensively studied over the last decades but only a small proportion of parasite species has been described. The total number of fish species on the Reef was given as 1625 by Hoese et al. [10]. The first estimates of parasite diversity on the Great Barrier Reef are by Rohde [11]. He based his estimates on the examination for monogenean parasites on the gills from altogether 550 fish of 74 species at one locality and 54 species at another, as well as on casual surveys for other parasites, and on comparison with surveys in other regions. He concluded that there must be at least 20,000 parasite species on fish at the southern end of the Great Barrier Reef alone. Later findings based on larger surveys of various groups more or less confirmed these dramatic conclusions. Thus, Justine [12] concluded that the number of parasites species on coral reef fish is at least ten times the number of fish species. Reviews of results for trematodes and monogeneans, groups studied best, were given by Cribb et al. [13] and Whittington [14]. |
| The number of trematode species was estimated to be about 1,100-1,800, unlikely to be below 1,000 or above 2,000 Cribb et al. [13] These estimates are based on the examination of 9,295 fish of 505 species and 60 families, of which 140 species were examined only once or twice. Whittington [14] states that only 85 monogenean species from fish had been described by 1998, from 40 of the most commonly caught fish. He estimates that the 3500 fish species found in Australian waters could harbor as many species of monogeneans., and that the 25,000 fish species worldwide could have 25,000 species of Monogenea, of which only about 3,000 – 4,000 had been described. But the total number estimated to exist may well be an underestimate, considering the strict specialization of monogenean species not only on particular host species but also sites on the host, which facilitates coexistence of many species even on one host individual. |
| For invertebrate parasites, Leung et al. [8] examining the quality and completeness of taxonomic data on aquatic (freshwater and marine) invertebrates, concluded that “there are very few examples of well-designed projects that specifically quantify the entire diversity of species in any major group”. |
| Concerning diversity in different habitats and oceanic regions, invertebrate hosts and their parasites in the deep-sea are practically unknown, as already pointed out by Rohde [6], with little advance made since [8]. Likewise, parasites of meiofaunal animals are more or less unknown, although species diversity in the meiofauna is very large. Thus, Armonies and Reise [15] reviewed the findings of the only area in which extensive studies of the meiofauna (small interstitial sand fauna) have been made over many years by many taxonomists, around the island of Sylt in the North Sea. 652 species had been recorded, 25 times as many as found in the macrofauna, and they estimated that a total of about 200 species still had to be described. No systematic survey of these meiofaunal animals for parasites has been made. |
| We conclude that the conclusions of Appletans et al. [3] that between one-third and two-thirds of marine species may be undescribed”, and that “If the current trends continues, most species will be described this century”, are unlikely to be correct at least for parasites. Future studies of marine diversity including that of parasitic species are urgently required. |
Biogeographical Patterns |
| Various geographical gradients demonstrated for marine parasites were most recently discussed in Rohde [16] and Leung et al. [8]. The main trends that have been investigated include latitudinal diversity gradients, latitudinal gradients in niche width including host range/specificity, latitudinal gradients in reproductive strategies, and longitudinal and depth gradients. There is an enormous number of studies on what is probably the clearest geographical trend in nature, that of latitudinal diversity gradients. Many groups of plants and animals in all ecosystems reach the greatest diversity at or near the equator, although some groups may have peaks elsewhere. Among marine parasites, both trematodes (largely endoparasitic flukes) and monogeneans (ectoparasitic flukes) have greatest species numbers at low latitudes. However, whereas increased diversity of the former is entirely due to the greater number of host species, tropical monogenean diversity is due to increased host numbers as well as greater numbers of monogenean species per host species. However, further studies using updated data from all latitudes are necessary to confirm this. Authors have still not reached consensus of what the underlying cause(s) of the gradients are. Rohde [17] listed 28 hypotheses that attempt to give an explanation. Gaston [18] concluded that “no single mechanism adequately explains all examples of a given pattern”. Nevertheless, there is convincing evidence from genetic studies of several plant and animal groups in support of the hypothesis of “effective evolutionary time”, which claims that increasing diversity with decreasing latitude is due to direct temperature effects on evolutionary rates [17]. This may be a primary cause but does not exclude the possibility that other factors may contribute as well, and that different explanations must be given for some groups exposed to certain environmental pressures (e.g, [19]). Recent reviews were given by Rohde [20] and Gillman and Wright [21] who gives summaries of work supporting the hypothesis. But again, more studies from additional groups are necessary to confirm the hypothesis. – Furthermore, the hypothesis also suggests that a rapid absorption of newly formed species into largely nonsaturated niche space occurs at all latitudes, i.e., that many ‘vacant niches’ exist [22,23] a terminology not accepted by some. This aspect of the hypothesis has been little considered and needs attention. The importance of the metabolic theory of ecology [24,25] for explaining distributional patterns of marine parasites has been discussed by Rohde [16]. It is well in agreement with the hypothesis of effective evolutionary time. |
| Latitudinal gradients in niche width have been examined for some marine parasites. Whereas host ranges (number of host species infected) of trematodes of marine fish is more or less the same at all latitudes, they are much smaller at low latitudes for monogeneans [16]. Stevens [26] formulated Rapoport’s rule, according to which latitudinal ranges of species are generally smaller in the tropics and wider at high latitudes. Numerous studies have provided evidence in favour of or against the hypothesis. It certainly is not a general phenomenon. There is convincing evidence that in many groups latitudinal ranges are in fact greatest in the tropics, decrease towards temperate/moderately cold regions and increase again at very high latitudes. Computer simulations using the Chowdury Ecosystem model have not provided any support for the rule [27,28]. Rohde [29] discussed it in the context of our knowledge of parasites, confirming these conclusions. |
| Diversity of those marine parasites that have been studied is generally greater in the Pacific than the Atlantic Ocean. - A latitudinal gradient in reproductive strategies has been well documented for marine mongeneans on the gills of fishes. Viviparous monogeneans of the family Gyrodactylidae predominate at high latitudes but are rare at low latitudes, which correspond to Thorson’s rule, according to which marine bottom invertebrates at high latitudes tend to produce small numbers of offspring, often by viviparity or ovoviviparity, and eggs and larvae which are large; larvae develop in egg capsules or by brooding (Mileikovsky [30]). |
| A well-documented longitudinal gradient has been documented for large coastal scombrid fishes. Greatest diversity is reached in tropical SE Asia, followed by a second center of diversity in the Caribbean. Species on the West coast of America are closely related to those in the Caribbean, but there has been no spreading across the East Pacific barrier, apparently because the fish cannot spread over the open ocean [16]. Depth gradients have been documented for many marine plant and animal groups, but little is known about them for parasites [31]. |
Conclusions |
| What should be do? Future perspectives |
| As we have seen, our knowledge of biodiversity of marine freeliving organisms but particularly their parasites is better for some groups than for others, but overall very poor. For example, very few studies have been made of deep-sea invertebrates and the meiofauna, and even fewer for their parasites. Larger parasites such as helminthes are better known than small protistan parasites. The number of species still to be described is probably in the 100,000s or even higher. Is there a need to describe them all? All we can hope for at this stage is to catalogue them and give numbers for different groups and habitats. Cum grano salis, a ‘species DNA machine’ would be useful into which we feed invertebrate samples and are rewarded by numbers of ‘types’ present in the sample distinguished by their DNA. Numbers of types or species even without descriptions would be extremely useful for finding further evidence for distributional patterns in the oceans. In particular, they would be useful for elucidating ecological patterns at the root of geographical ones. How many empty niches are there (or for those who do not like the term vacant niche: how many new species can be accommodated in a habitat or ecosystem without displacing or compressing the niches of other species)? Is the hypothesis of effective evolutionary time generally valid? – Finally, ecology and biogeography rely to a large degree on the analysis of correlations to find “explanations” of patterns. Needed, however, is the uncovering of mechanisms that give causal explanations of patters? Such a mechanism is, for example, temperature effects on mutation rates, generation times and speed of selection, as postulated by the hypothesis of effective evolutionary time as an explanation of latitudinal gradients in diversity. Useful also are agent-based models, such as the Chowdury Ecosystem Model, which permits examination of evolutionary factors by computer simulations. |
| Few such studies have been made [27,28,32]. Many parasites have complex life cycles involving several hosts, which have to be known if we want to understand their role in ecosystems. Of the hundreds of trematode species on the Great Barrier Reef, life cycles are known for only two [13]. Important for understanding how ecosystems work and therefore how distributional patterns come about, are studies of how organisms find their place in the system, i.e. how do they orientate, find hosts etc.? Many mechanisms are usually involved, but I am aware of only a single study that has demonstrated reactions to magnetic stimuli in marine parasites, that by Rothsey and Rohde [33]. Magnetic orientation could play an important role in how animals locate hosts and needs much further attention. |
References |
|
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi