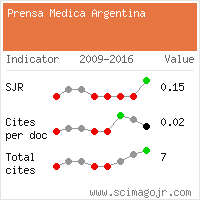
Opinion Article, La Prensa Medica Vol: 2 Issue: 109
Emerging Strategies for Protein-Protein Interaction Inhibitor Design
Michelle Rhinehart*
1Department of Pharmaceutical Chemistry, University of California at San Francisco, San Francisco, California, USA
*Corresponding Author: Michelle Rhinehart,
Department of Pharmaceutical
Chemistry, University of California at San Francisco, San Francisco, California,
USA
E-mail: rhinehartm98@ucsf.edu
Received date: 25 April, 2023, Manuscript No. LPMA-23-102751;
Editor assigned date: 27 April, 2023, PreQC No. LPMA-23-102751(PQ);
Reviewed date: 12 May, 2023, QC No. LPMA-23-102751;
Revised date: 19 May, 2023, Manuscript No. LPMA-23-102751(R);
Published date: 26 May, 2023, DOI: 10.4172/0032-745X.1000163
Citation: Rhinehart M (2023) Emerging Strategies for Protein-Protein Interaction Inhibitor Design. La Prensa Medica 109:2.
Abstract
Description
Protein-Protein Interactions (PPIs) are important for numerous biological processes and are involved in the development and progression of various diseases. Targeting PPIs with small molecule inhibitors has long been a challenge in drug discovery due to the dynamic nature of protein interfaces. However, recent advancements in technology, computational modeling, and structural biology have paved the way for the emergence of innovative strategies in PPI inhibitor design. This study provides an overview of the emerging strategies and approaches that are revolutionizing the field of proteinprotein interaction inhibitor design.
Computational methods have significantly contributed to the design of PPI inhibitors. Molecular docking, virtual screening, and molecular dynamics simulations are powerful tools used to predict and analyze protein-protein interactions. Structure-based virtual screening enables the screening of large compound libraries to identify potential inhibitors that can target specific PPI interfaces. Molecular dynamics simulations provide valuable insights into the dynamics and stability of PPIs, aiding in the design of allosteric inhibitors that modulate PPIs indirectly.
Peptide-based inhibitors offer an alternative approach to target PPIs. Advances in peptide chemistry and modification techniques have led to the development of peptide mimetics and stapled peptides. Peptide mimetics mimic the secondary structure of key interaction motifs and can be optimized for improved stability and affinity. Stapled peptides, incorporating chemical cross-linking, enhance their proteolytic stability and cell permeability. These strategies have shown possibility in targeting challenging PPIs, such as those involved in protein-protein interfaces that lack druggable pockets. Macrocyclic and constrained peptides are emerging as powerful tools in PPI inhibitor design. These peptides exhibit enhanced stability and selectivity due to their constrained structures, which restrict conformational flexibility and improve binding affinity.
Techniques like peptide cyclization, stapling, and introduction of non-natural amino acids enable the design of macrocyclic and constrained peptides that can effectively disrupt PPIs. Fragment-based approaches involve screening small, low molecular weight compounds to identify fragment hits that bind to specific regions of PPI interfaces. These fragment hits are then elaborated and optimized through structure-guided medicinal chemistry to develop potent PPI inhibitors. Fragment-based methods allow exploration of a broader chemical space and provide valuable starting points for inhibitor design.
Protein-degradation technologies, such as Proteolysis-Targeting Chimeras (PROTACs) and Specific and Non-genetic IAP (Inhibitor of Apoptosis Protein)-dependent protein erasers (SNIPERs), offer a unique approach to modulate PPIs. PROTACs recruit E3 ubiquitin ligases to target disease-associated proteins for proteasomal degradation. SNIPERs, on the other hand, recruit IAPs to induce the degradation of target proteins. These degradation technologies allow selective removal of disease-relevant proteins, providing a novel avenue for therapeutic intervention.
Multivalent inhibitors present a promising strategy to enhance binding affinity and selectivity in PPI inhibition. By presenting multiple binding motifs or ligands, multivalent inhibitors can simultaneously engage multiple protein interfaces, leading to stronger and more specific interactions. This approach allows targeting complex PPIs with higher affinity and potentially improved therapeutic outcomes.
Conclusion
The emerging strategies discussed in this article demonstrate the innovative approaches being pursued in protein-protein interaction inhibitor design. Computational methods, peptide-based inhibitors, macrocyclic and constrained peptides, fragment-based approaches, protein-degradation technologies, and multivalent inhibitors all contribute to expanding the toolbox for targeting PPIs and hold great potential for future drug discovery and therapeutic interventions.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi