Research Article, Lpmj Vol: 106 Issue: 4
Evaluation of some bone biomarkers in thyroid diseases patients
Mohammad Ahmad Abdalla1 Biology Department, College of Sciences, Tikrit University, Iraq
2 Clinical Biochemistry Unit, Salah Aldeen Health Directorate, Iraq
3 Anatomy Department, College of Medicine, Tikrit University, Iraq
*Corresponding Author : Mohammad Ahmad Abdalla
Department of Human Anatomy,
University College of Medicine, Iraq
Tel: +964702758383
E-mail: dr.mohammad68@tu.edu.iq;
Received Date: July 02, 2020; Accepted Date: July 11, 2020; Published Date: July 29, 2020
Citation: Mohammad Ahmad Abdalla (2020) Evaluation of some bone biomarkers in thyroid diseases patients LPMJ 106:4.
Abstract
Thyroid hormones are key regulators of bone homeostasis, and Wnt signaling has been implicated in thyroid hormone-associated bone loss. Bone remodeling is a complex process carried out by the intricate interactions of osteoclasts and osteoblasts in the bone microenvironment. Serum samples were collected from 90 individuals, 30 of these individuals were normal and 60 cases subdivided into two groups hyper and hypothyroidism (30 patients for each group) which thyroid patients visited to privet clinic in Tikrit city from June 2019 to November 2019. Patients and controls that aged from (18-50) year had assay of Osteocalcin , Sclerostin , Osteoprogestrin , T3, T4 and TSH. This study showed that levels of (T3, T4 and SOST) increased in hyperthyroidism patients rather than control, while levels of (TSH, OC and OPG) were decreased. On the other hand, the levels of (T3, T4, OC and OPG) were decreased in hypothyroidism patients rather than control, but levels of (TSH and SOST) were increased. Osteocalcin and Osteoprogestrin are decreased in thyroid diseases but SOST is increased rather than control, therefore, thyroid hormones disturbance effect on bone metabolism and changed some bone biomarkers.
Keywords: Osteocalcin , Sclerostin , Osteoprogestrin , Thyroid diseases
Introduction
The hypothalamic–pituitary–thyroid axis plays a key role in skeletal development, acquisition of peak bone mass and regulation of adult bone turnover. Euthyroid status is essential for maintenance of optimal bone mineralization and strength. In population studies, hypothyroidism and hyperthyroidism have both been associated with an increased risk of fracture[1].
The thyroid gland predominantly secretes the pro-hormone T4 but also produces smaller amounts of the active hormone T3. The majority of circulating T3 is generated via 5 ´-deiodination of T4 by the type 1 and 2 iodothyronine deiodinase enzymes (D1 and D2) [2].
Altered thyroid status has well-known and profound effects on skeletal development and growth and on adult bone maintenance. The established view that these effects are solely a consequence of altered T3 action in skeletal cells has been questioned however. Studies of TSHR deficient mice have resulted in the proposal that TSH is a direct inhibitor of bone turnover, acting via the TSHR expressed in osteoblasts and osteoclasts [3].
Effects of thyroid dysfunction on the bone remodelling cycle have been investigated in several studies in adults[4]. In hypothyroidism the duration of the remodelling cycle is increased: the duration of the osteoclastic resorption phase is extended 2-fold, whereas the time for osteoblastic bone formation and secondary mineralization is prolonged 4-fold. These changes result in low bone turnover and an overall gain in bone mass and mineralization. By contrast, bone resorption and formation are both accelerated in hyperthyroidism and the remodelling cycle is shortened: the duration of the resorption phase is reduced by 60% and the formation phase is shortened by 30%. The frequency of initiation of bone remodelling is also markedly increased and bone resorption is increased to a greater extent than bone formation. The net result of these changes is a 10% loss of bone per remodelling cycle in established hyperthyroidism leading to high bone turnover osteoporosis. All these features are also observed in patients with thyrotoxicosis due to Graves’ disease, which results from persistent activation of the TSHR in thyroid follicular cells by TSHRstimulating antibodies [5].
Sclerostin is a glycoprotein, which is mainly produced in osteocytes. It is a product of sclerostin gene and it reduces bone formation in osteoblasts by inhibiting Wnt signaling pathway. In wild type mice with avoided mechanical loading, demonstrated increased synthesis of sclerostin in opposition to decreased Wnt/β-catenin signaling. However, the bone loss detected after reduced mechanical loading was not identified in sclerostin negative mice [6].
Since thyroid hormones and sclerostin have an influence on bone tissues via Wnt signaling pathway, it seemed reasonable to search both of them in hyperthyroidism patients. The relation between sclerostin and thyroid hormones and the relation of both, with bone metabolism markers such as serum OC and OPG for bone resorption were investigated [7].
Osteoprotegerin (OPG) and its cognate ligand, receptor activator of nuclear factor-kappa ligand (RANKL), have been identified as important factors involved in the regulation of bone metabolism mediating the paracrine signaling between osteoblast and osteoclast [8]. OPG acts as decoy receptor binding RANKL and thereby inhibiting the interaction between RANKL and its receptor on the osteoclast surface [9]. In humans, OPG has been implicated in the pathogenesis of postmenopausal osteoporosis [10] and other metabolic diseases characterized by bone loss [11].
Hyperthyroidism masked the physiological age-dependent increase in OPG production [12]. Previous study shows that (1) hyperthyroidism is accompanied by an increase in serum OPG concentrations in relation to the excess in thyroid hormones and accelerated bone turnover; (2) serum OPG concentrations are higher in patients with Graves’ disease (GD) in comparison with patients with toxic nodular goiter (TNG); and (3) the treatment of hyperthyroidism produces decrease in serum OPG concentrations in temporal relationship with the normalization of bone metabolism (Amato G 2004).
Osteocalcin, usually employed as bone-forming parameter during bone turnover, was synthesized and secreted by osteoblasts, stored in the bone matrix and can be released into blood. In condition of high bone turnover such as postmenopausal women and hyperthyroidism patients, serum osteocalcin will elevate. Hyperthyroidism is the most common clinical model with high bone turnover [13].
Materials and Methods
(Five-Ten) ml of venous blood was drawn from thyroid diseases and control subjects allowed it to clot in plain tube at room temperature. The serum was aspirated after centrifugation at (3000 rpm) for 30 minutes, then divided into aliquots in plastic tubes and stored at (20 °C) until the time of estimation. Serum samples were collected from 90 individuals, 30 of these individuals were normal and 60 cases subdivided into two groups hyper and hypothyroidism (30 patients for each group) which thyroid patients visited to privet clinic in Tikrit city from June 2019 to November 2019. Patients and controls that aged from (18-50) year had assay of Osteocalcin , Sclerostin , Osteoprogestrin , T3, T4 and TSH. All these control individuals are non-diabetic and non-smoker with no familial history of diabetes or personal history of hypertensive, thyroid, thalassaemia or renal diseases. Serum (T3, T4 and TSH) were determined by using AccuBind ELISA Microwells (sandwich enzyme immunoassay kit) (Monobind Inc., USA). Osteocalcin, Sclerostin and Osteoprogestrin were measured by using Enzyme Linked Immunosorbent Assay (ELISA) kits (Sunlong Company). The statistical analysis was carried out by using statistical program (SPS, 2001) and comparison between groups which were made by using one-way analysis of variance (ANOVA), and tried out the arithmetic means for parameters by using test of Duncan multiple ranges. The level of statistical significance was taken at (P˂0.05).
Result
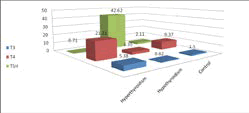
The mean±SD of thyroid hormones levels were (T3= 5.38±2.23 ng/ ml, T4= 21.31±2.49 ug/dl and TSH= 0.71±0.4 IU/ml) in patients with hyperthyroidism, but for hypothyroidism patients were (T3= 0.62±0.47 ng/ml, T4= 3.35±1.21 ug/dl and TSH= 42.62±25.64 IU/ml) while in normal subjects were (T3= 1.50±0.28 ng/ml, T4= 9.37±1.49 ug/dl and TSH= 2.11±0.58 IU/ml). Serum OC in hyperthyroidism patients was (2.59±0.63) pg/ml, but for hypothyroidism patients was (3.74±2.13) pg/ml while in normal subjects was (4.0±1.9) pg/ ml. SOST level was (23.28±6.75) ng/ml in hyperthyroidism, but for hypothyroidism patients was (40.75±26.71) ng/ml while in normal subjects was (22.6±5.0) ng/ml. OPG level in hyperthyroidism patients was (77.24±14.98) pg/dl but for hypothyroidism patients was (148.14±83.01) pg/dl while in normal subjects was (160.7±103.4) pg/dl (as shown in Table 1and fig 1and 2).
| Control | P value | Hypothyroidism | P value | Hyperthyroidism | Parameters |
|---|---|---|---|---|---|
| 30 | 30 | 30 | Number of subjects | ||
| (18-48) | (18-48) | (18-48) | Age (year) | ||
| 1.50±0.28 | P≤0.01 | 0.62±0.47 | P≤0.01 | 5.38±2.23 | T3 ng/ml |
| 9.37±1.49 | P≤0.01 | 3.35±1.21 | P≤0.01 | 21.31±2.49 | T4 ug/dl |
| 2.11±0.58 | P≤0.01 | 42.62±25.64 | P≤0.01 | 0.71±0.4 | TSH IU/ml |
| 4.0±1.9 | P>0.05 | 3.74±2.13 | P≤0.05 | 2.59±0.63 | OC pg/ml |
| 22.6±5.0 | P≤0.05 | 40.75±26.71 | P>0.05 | 23.28±6.75 | SOST ng/ml |
| 160.7±103.4 | P>0.05 | 148.14±83.01 | P≤0.01 | 77.24±14.98 | OPG pg/dl |
Table 1: The mean±SD for all parameters in hyper and hypothyroidism patients and control.
Discussion
This study showed that the levels of (T3 and T4) with highly significant difference (P≤0.01) and SOST with no significant difference (P>0.05) increased in hyperthyroidism patients rather than control, while the levels of (TSH and OPG) with highly significant difference (P≤0.01) and OC with significant difference (P≤0.05) were decreased. On the other hand, the levels of (T3 and T4) with highly significant difference (P≤0.01) and (OC and OPG) with no significant difference (P>0.05) were decreased in hypothyroidism patients rather than control, but levels of TSH with highly significant difference (P≤0.01) and SOST with significant difference (P≤0.05) were increased.
Thyroid hormones are important regulators of the skeleton that stimulate both the formation and resorb¬tion of bones and cause thinning in the mineralized bones (Rija FF 2019). It has been stated in the literature that the thyroid hormones (O’Shea P J 2012, Tsourdi E 2015) and sclerostin were influential on bone tis¬sue by Wnt/β- catenin signal pathway (Wang L 2007, Winkler DG 2003, Poole KE 2005).
Osteocalcin is a Gla-protein of the bone matrix, synthesized from mature osteoblasts and hypertrophic chondrocytes. Unfortunately, osteocalcin is not only localized in cells at minerali¬zed surfaces since it was reported that both osteocal¬cin mRNA and protein are synthetized at bone mar¬row megakaryocytes, peripheral blood platelets and at bone marrow adipocytes. The blood levels of intact osteocalcin show the bone formation theoretically. The release of immunoreactive fragments of osteocalcin to the blood is related with bone resorbtion process [14]. Lee and coworkers (2000) reported that the ma¬jority of the osteocalcin is stored in extracellular bone matrix, and the osteocalcin in serum is the non-ab¬sorbed part of total osteocalcin with hydroxyapatite [15].
Recent studies provide evidence that the RANKL/OPG system might play a role in the crosstalk between thyroid gland and bone [16]. Whether thyroid gland acts as a source of RANKL/OPG production is not known. Both immunohistochemical and mRNA expression studies revealed that OPG/RANKL are expressed in both malignant and benign thyroid tissues[17]. Immune cells are capable of producing and interacting with these mediators. For example, dendritic cells express RANK/RANKL, which regulate the important interactions between T cells and dendritic cells that play important roles in thyroid autoimmunity [18].
Conclusion
Osteocalcin and Osteoprogestrin are decreased in thyroid diseases rather than control. SOST is increased in thyroid diseases rather than control, but all bone biomarkers lower in hyperthyroidism rather than hypothyroidism, therefore, thyroid hormones disturbance effect on bone metabolism.
References
- Gogakos AI, Bassett JD, Williams GR. Thyroid and bone. Archives of biochemistry and biophysics 2010;503(1):129-36.
- Bianco A C, Salvatore D, Gereben B, Berry M J, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. 2010. 23:38–89.
- Abe E, Marians R C, Yu W, Wu X B, Ando T, Li Y, Iqbal J and et al. TSH is a negative regulator of skeletal remodeling. Cell 2010;115(2):151-62.
- Hussein S Z, Ali S J. Evaluation of Serum Thyroid Hormones and Cortisol Level in Patients with Chronic Renal Failure. 1st scientific conference of medical group colleges 2013. 26-27:32-7.
- Davies T F, Ando T, Lin R Y, Tomer Y, Latif R. Thyrotropin receptor–associated diseases: from adenomata to Graves disease. J. Clin. Invest. 2005;115, 1972-83.
- Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y and et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/β-catenin signaling. J Bone Miner Res. 2009;24:1651–61.
- Sarıtekin İ, Acikgoz Ş, Bayraktaroğlu T, Kuzu F, Can M, Güven B, Mungan G and et al. Sclerostin and bone metabolism markers in hyperthyroidism before treatment and interrelations between them. Acta Biochimica Polonica 2017;64(4):597-602.
- Rija F F, Almahdawi Z M, Hussein S Z. Correlation between Calcium Sensing Receptor with other Calcium Regulators in Osteoporosis and Osteomalacia Patients. Plant Archives 2019;19(1):1199-207.
- Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001;79:243–53.
- -Bekker PJ, Holloway D, Nakanishi A, Arrighi M, Leese PT, Dunstan CR. The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res. 2001; 16:348–60.
- Ueland T, Bollerslev J, Godang K, Muller F, Froland SS, Aukrust P. Increased serum osteoprotegerin in disorders characterized by persistent immune activation or glucocorticoid excess—Possible role in bone homeostasis. Eur J Endocrinol 2001;145:685–90.
- Kudlacek S, Schneider B, Woloszczuk W, Pietschmann P, Willvonseder R, Austrian Study Group. Normative values of bone metabolism serum levels of osteoprotegerin increase with age in a healthy adult population. Bone 2003;32:681–6.
- Zhong N, Xu B, Cui R, Xu M, Su J, Zhang Z, Liu Y and et al. Positive correlation between serum osteocalcin and testosterone in male hyperthyroidism patients with high bone turnover. Experimental and Clinical Endocrinology & Diabetes 2016;124(7):452-6.
- Zaitseva OV, Shandrenko SG, Veliky MM. Biochemical markers of bone collagen type I metabolism. Ukr Biochem J. 2015;87:21-32
- Lee AJ, Hodges S, Eastell R. Measurment of osteocalcin. Ann Clin Biochem. 2000; 37:432-46.
- Ma R, Morshed S, Latif R, Zaidi M, Davies TF. The influence of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on osteoclastogenesis. Thyroid : Off J AmThyroid Assoc. 2011; 21(8):897-906.
- Heymann MF, Riet A, Le Goff B, Battaglia S, Paineau J, Heymann D. OPG, RANK and RANK ligand expression in thyroid lesions. Regul Pept. 2008; 148(1-3):46-53.
- Sood SK, Balasubramanian S, Higham S, Fernando M, Harrison B. Osteoprotegerin (OPG) and related proteins (RANK, RANKL and TRAIL) in thyroid disease. World J Surg. 2011; 35(9):1984-92.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi