Market Analysis, J Genet Disor Genet Rep Vol: 9 Issue: 1
Market Analysis of Genome Editing and Gene Therapy
William J. Rowe
Former Assistant Clinical Professor of Medicine Medical University of Ohio at Toledo USA, E-mail: rowefemsinspace@gmail.com
Keywords: Genome Editing
Market Analysis
The global genome editing market is expected to reach $3,514.08 Million by 2019 from $1,845.25 Million in 2014, at a CAGR of 13.75% between 2014 and 2019. Factors such as rapid increase in the number of pharmaceutical and biotechnology industries, strong trend of R&D in life science research, increasing government funding for genomics, technological advancements, increased demand for synthetic genes, and the overall rise in the production of genetically modified crops are driving the growth of the genome editing market. On the other hand, stringent regulatory policies and ethical issues are major factors restraining the growth of this market.
The worldwide market for incited pluripotent stem cells or iPSCs was assessed to add up to $853 million of every 2012 and near $1.2 billion out of 2013, averaging 40% development. The market is relied upon to reach $2.9 billion out of 2018, an expected compound yearly development rate (CAGR) of 19.7% for the five-year time frame 2013 to 2018.
Rivalry is as of now extraordinary among organizations endeavoring to make mark mindfulness. This is hence, convincing the market players to plan advertise based procedures. Cold blood stem cell is the main kind of stem cell that is put away in controlled condition because of its lower volume higher cell count highlight. Capacity benefit showcase is normal develop at 33.4% CAGR from 2013-2020.
The U.S. advertise for regenerative drug items utilized just in bone and joint applications is relied upon to develop at a compound yearly development rate (CAGR) of 6.8% from $2.9 billion of every 2015 to about $4.1 billion of every 2020.
Worldwide regenerative medications showcase is anticipated to reach $30,237 million by 2022. Cell treatment section is relied upon to command the market all through the estimate time frame. North America drove the worldwide market in 2015, and is relied upon to stay overwhelming amid the 2015-2022.
Importance and Scope of Cell Therapy & Molecular Medicine
Cell Therapy describes about the administration of live whole cells or maturation of a specific cell population in a patient for treatment of a disease. In recent times, cell therapy is expanding its range of cell types for administration. Various treatment strategies of cell therapy include, isolation and transfer of specific stem cell populations, administration of effector cells, induction of mature cells to become pluripotent cells, and reprogramming of mature cells. By administrating a large number of effector cells has benefited a large number of cancer patients, transplant patients with unresolved infections, and patients with chemically destroyed stem cells in eyes. Gene therapy on the other hand is expressed as an experimental treatment that involves introduction of genetic
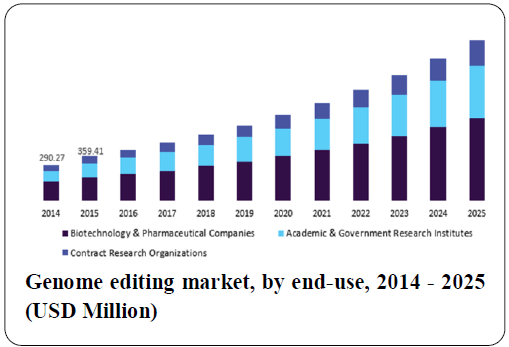
material into the cells in order to correct the defective genes. Retroviruses and adenoviruses are among the most commonly used viral vectors in trials for treating diseases under gene therapy. Other viral vectors such as adeno-associated viruses, pseudotyped viruses and herpes simplex viruses are also gaining popularity. Some proposed non viral vectors for gene transfer include physical methods and the employment of chemical vectors like lipoplexes, polyplexes and inorganic nanoparticles. Several diseases benefit from both cell and gene therapies. For example, some patients have a severe combined immunodeficiency disease (SCID) but unfortunately do not have a suitable donor of the bone marrow. Several dozen of patients have been treated with both combined cell and gene therapy approach. More evidence for wider clinical applications for both cell and gene therapy are expected as more cell and gene therapy studies progress from preclinical phase to clinical trial. Molecular Medicine is one of an important application in the field of bioinformatics research. Due to the fact that complete sequencing of human genome has enabled to improve the biological reserach and clinical medicine, scientists can now find cures against diseases either heriditary or acquired. Therefore, it will enable the pharmaceutical industry to design drugs which will only target those genes which are defective.
Why Osaka, Japan?
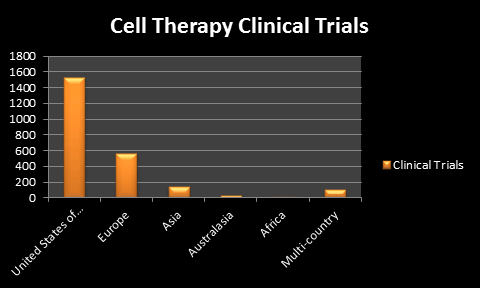
As both cell and gene therapy has gained many applauds from all over the world, and due to this reason these therapies are given much more importance from the beginning of their inceptions. Cell and Gene therapies both are well performed worldwide in order to treat many types of diseases like SCID, Cancer, Cardiovascular diseases Type 1 diabetes and many more. In spite of many legal and ethical issues these therapies are now accepted worldwide but still faces many challenges in some countries and this is the reason that many people are still today out of reach of their benefits especially on human health. According to ClinicalTrials.gov, Asia has conducted maximum number of studies (2,740) among other countries which shows a huge scope for promoting the benefits of both cell and gene therapy by the scientists, researchers and clinical industries to treat many diseases which do not have a proper treatment till date. Gene therapy products have not been currently approved by US FDA and therefore gene therapy products are still in the investigational stage. Some of the promising treatments that are not yet licensed are mainly available
Societies associated with Cell Therapies
• Adult Stem Cell Research Network
• Alzheimer’s Cure Foundation
• American Academy of Anti-Ageing Medicine (A4m)
• American Association for Cancer Research (AACR)
• American Cancer Society
• American Society for Cell Biology
• American Society for Investigating Pathology
• American Society Radiation Oncology
• Cell Therapy Foundation
• European Society of Gene and Cell Therapy
Societies associated with Gene Therapies
• American Society of Gene and Cell Therapy (ASGCT)
• Australasian Gene and Cell Therapy Society (AGCTS)
• Austrian Network for Gene Therapy
• British Society of Gene Therapy
• European Society of Gene and Cell Therapy (ESGCT)
• French Society of Cellular and Gene Therapy (SFTCG)
• German Gene Therapy Society (DGGT)
• International Society for Cancer Gene Therapy (ISCGT)
• Irish Society for Gene & Cell Therapy (ISGCT)
• Japan Society for Gene Therapy (JSGT)
Market Research on Cell Therapy
The market of cell therapy in terms of revenues is $3 billion and the annual growth rate from 2011- 2016 is 28.4%. The market of stem cell therapy is poised to grow at a CAGR of 39.5% from 2015 to 2020., to reach $330 million by 2020. The global stem cell therapy market on the basis of the mode of treatment is segmented into allogenic and autologous stem cell therapy.
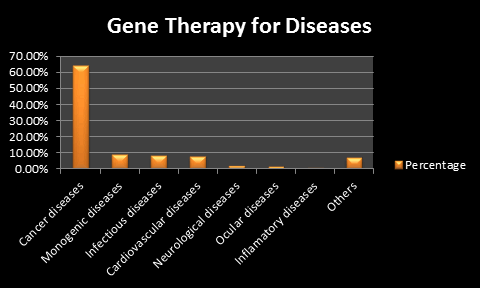
Market Research on Gene Therapy
The gene therapy market in spite of presenting few marketable products and being nascent in terms of revenue generation holds tremendous growth potential. As per a new estimation carried out in a latest study, the global gene therapy industry has the potential to become a multi-million dollar industry by the end of 2017 as new products, especially those in the advanced stage of clinical studies or with pending approvals, may enter the market to boost the growth.
Companies associated with Cell Therapy
• Cynata Therapeutics
• CellTherapies
• Living Cell Technologies
• Mesoblast
• Regeneus
• Ascend Biopharmaceuticals
• Activartis
• Aposcience
• Bone Therapeutics
• PluriCell
Companies associated with Gene Therapy
• Addgene
• Advanced Cell & Gene Therapy, LLC
• Advanced Vision Therapies, Inc
• AlphaVax Human Vaccines, Inc
• Altogen Biosystems
• Applied Tissue Technologies LLC
• Ark Therapeutics Ltd
• Asklepios BioPharmaceutical Inc
• Athersys, Inc
• Austrianova
-
5th International Conference on Genome Editing and Gene Therapy
August 19-20, 2020 Osaka, Japan
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 



