Research Article, J Food Nutri Disor Vol: 9 Issue: 4
Specialized Multivitamin Supplementation is Needed After Roux-en-Y Gastric Bypass Surgery
Astrid Van Eijgen1*, Greet Vanheule2, Wim Bouckaert1, Liesbeth Decoutere1 and Mieke Van Den Driessche1
1VZW Jessa Ziekenhuis Hasselt Salvatorstraat, Belgium
2Metagenics Europe E. Vlietinckstraat, Belgium
*Corresponding Author : Astrid Van Eijgen
VZW Jessa Ziekenhuis Hasselt Salvatorstraat, Zorgcluster Apotheek-CSA, Europa Ziekenhuizen VZW, Site St(e)-Elisabeth, De Frelaan 206-1180, Bruxelles
E-mail: astridvaneijgen@hotmail.com
Received: August 27, 2020 Accepted: September 20, 2020 Published: September 28, 2020
Citation: Eijgen VA, Vanheule G, Bouckaert W, Decoutere L, Driessche MVD (2020) Specialized Multivitamin Supplementation is Needed After Roux-en-Y Gastric Bypass Surgery. J Food Nutr Disor 9:4. doi: 10.37532/jfnd.2020.9(4).283
Abstract
Roux-en-Y gastric bypass (RYGB) leads to a restricted absorption and decreased intake of nutrients making these patients very vulnerable for the development or worsening of nutritional deficiencies. The present study evaluates the effectiveness of supplementation with a specialized Multivitamin (MVM) in comparison with a standard multivitamin.
An open label, randomized, 12 month study was conducted comparing a specialized multivitamin in combination with 1000 mg Calcium, 1000 IU vitamin D and 28 mg elemental iron for premenopausal women (<45y) with a standard multivitamin supplement. Severe vitamin D deficiencies were treated in both groups with a drinkable ampule of 25.000 IU vitamin D.
In total 145 patients underwent a RYGB of which 91 patients participated in the intervention group (specialized MVM) and 54 in the control group (standard MVM). The intervention group was analyzed prospectively while the standard group was analyzed retrospectively. Baseline characteristics were similar for both groups. Per protocol analysis demonstrated significant higher serum Vitamin B12 levels (p<0.001) for the intervention group. The control group even had a significant reduction of vitamin B12 concentrations over time (p<0.001). In addition, the intervention group showed higher vitamin D levels after surgery, even higher than the control group (p<0.001), although more patients received the D-cure in the control group (45% at 4 months and 26% at 7 months in comparison with 9% and 11% for the intervention group).
To conclude, a specialized MVM with adjusted doses of vitamins and minerals is needed to resolve and/or prevent deficiencies after RYGB, especially vitamin B12 and vitamin D.
Keywords: Vitamins; Nutrients; Deficiency, Vitamin B12; Roux-en-Y gastric bypass
Introduction
Roux-en-Y Gastric Bypass (RYGB) in combination with nutritional advice and an adapted life style is recognized as one of the most efficient interventions for substantial weight loss in case of morbid obesity [1]. However, this surgery impairs the absorption of vitamins, proteins and minerals, due to the change in anatomy and physiology [2,3]. Important factors are the small stomach pouch, the limited contact of food with stomach fluids (HCl and pepsin) and the more distal attachment of the alimentary limb which results in a decreased contact period between food, intestinal mucosa and digestive fluids (chymotrypsin, trypsin, peptidases) [4]. Beside a disturbed absorption, changes in hunger and satiety feelings are related to the diminished intake of proteins and weight loss [5]. As most morbidly obese patients have a deficient nutritional status before bariatric surgery, the risk on health complications increases after surgery due to the development and/or worsening of nutritional deficiencies [3,6-11].
Frequently observed deficiencies are iron, vitamin B12 and calcium leading respectively to anemia and osteoporosis in the long term [1,12,13]. Also shortages in fat-soluble vitamins like vitamin D, A, E and K are reported [13]. Due to the high prevalence of nutritional deficiencies after bariatric surgery, monitoring and micronutritional supplementation are essential [14-17]. As a consequence, a multivitamin preparation is recommended for life and premenopausal women (<45 y) are advised to take additional iron supplements since a reduced absorption of iron, folic acid and cobalamin can lead to anemia [18-22]. Global vitamin deficiencies can lead to numerous general complaints such as fatigue, vomiting, nausea, hair loss, dizziness, night blindness, dry skin, dry mouth, headaches, loss of sensation, loss of taste and smell, etc. [23]. Also neurological complications are described in literature such as peripheral and optical neuropathy and myelopathy, mainly caused by vitamin B deficiencies [24,25].
Standard over-the-counter multivitamin and mineral supplements do not contain the adequate amounts of certain vitamins and minerals since bariatric patients require tailored supplements to maintain an optimal nutritional status for life [17,23]. Hence, additional supplementation in higher dosages may be necessary to prevent deficiencies [18,19,26,27]
This open label study compares the daily intake of a specialized multivitamin preparation specifically developed for bariatric patients, with a standard multivitamin preparation. The primary endpoint is the change in lab values from baseline (2-4 weeks before surgery) to 4 and 7 months post-surgery.
Materials and Methods
The study protocol was approved by the Medical Ethics Committee of University hospital Leuven and by the local Ethics Review Committee of Jessa Hospital in Hasselt, Belgium. Patients are recruited by the bariatric surgeon, the obesity coordinators or a clinical pharmacist linked to the Obesity Center of the Jessa Hospital. Informed consent was obtained from all individual participants included in the study. The study was conducted according to ICH GCP, the declaration of Helsinki and local regulatory guidelines.
Study design
The study was a monocenter, open label, nonblinded pro-and retrospective cohort study. Patients were categorized in 2 groups, taking 2 different multivitamin supplements starting 3 days to 7 days following the primary laparoscopic RYGB, for 1 year. Lab values were evaluated in the defined periods: 4 to 2 weeks before surgery (baseline) and 3-4, 6-7 and 12 months after surgery. These periods are hereafter mentioned as baseline or Pre-OP, 4 and 7 months post-surgery.
Patients
Patients were selected according to their LRYGB surgery time point. The control group was retrospectively analyzed while the intervention group was prospectively analyzed. Included participants were older than 18y, had a Body Mass Index (BMI)>40 kg/m² or a BMI >35 kg/m2 combined with at least one obesity related co-morbidity. Patients with a history of bariatric surgery or secondary laparoscopic RYGB surgery, postoperative complications impairing the oral intake of supplements, not speaking or understanding the national languages or without obtained consent to participate in the trial were excluded to participate. Termination could be due to withdrawal of consent, quitting the trial or lost to follow-up. Surgical procedure: All patients underwent a standard laparoscopic RYGB surgery which involves a stapled pouch (25-30 cc) and a linear gastro-enterostomy with an alimentary canal of 100 cm. A banded bypass has never been executed. Pre-surgery, deficiencies were corrected if present. Post-surgery, all patients received Low Molecular Weight Heparin (LMWH) (nadroparin-5700 IU anti-Xa/day) for one month and a proton pomp inhibitor (pantoprazole 40 mg/day) for three months, as part of the standard post-operative protocol.
Intervention and control
In the intervention group, BariNutrics® Multi was taken once daily. BariNutrics Multi® is a specialized supplement specifically developed to meet the needs of bariatric patients. This supplement contains high amounts of vitamins and minerals, with most of them in higher doses than the Recommended Daily Allowance (RDA) (e.g. vitamin B1, B6, B12, D3, K, B9). In addition, also BariNutrics® Calcium, a combined preparation of 500 mg calcium citrate and 500IU cholecalciferol (Vitamin D3) was taken twice a day resulting in a daily total intake of 1000 mg elemental calcium and 3000IU vitamin D. Premenopausal women (<45 y) received extra iron supplementation, BariNutrics® Iron containing 90 mg iron fumarate or 28 mg elemental iron resulting in a total daily intake of 42 mg elemental iron.
This treatment is seen as the standard treatment after bariatric surgery, according to international recommendations [28]. They advise supplementation with a specialized multivitamin in combination with calcium and vitamin D and extra iron when necessary. In the control group, a standard multivitamin, Omnibionta® integral, was given once daily. No extra iron or calcium was standardly prescribed in this group. The composition of both supplements can be found in Table 1.
| Ingredients | Form | Dosage | RDA (%) | Dosage | RDA (%) |
|---|---|---|---|---|---|
| Vitamin A | Retinolpalmitate and | 800 µg | 100 | 800 µg | 100 |
| beta-carotene | |||||
| Vitamin B1 | Thiamin mononitrate | 4.2 mg | 382 | 2.8 mg | 225 |
| Vitamin B2 | Riboflavin | 2.8 mg | 200 | 3.2 mg | 229 |
| Vitamin B3 | Niacinamide | 32 mg | 200 | 36 mg | 225 |
| Vitamin B5 | Calcium panthotenate | 12 mg | 200 | 12 mg | 200 |
| Vitamin B6 | Pyridoxal-5-phosphate | 2.8 mg | 200 | 2 g | 143 |
| Vitamin B12 | Methylcobalamine | 500 µg | 20.000 | 1 µg | 40 |
| Vitamin C | Ascorbic acid | 160 mg | 200 | 180 mg | 225 |
| Vitamin D3 | Cholecalciferol | 50 µg (2000 IE) |
1000 | 5 µg (200 IE) |
100 |
| Vitamin E | Mix of natural tocopherols and tocopherol acetate | 24 mg | 200 | 10 mg | 83 |
| Vitamin K | Phytomenadione | 135 µg | 200 | / | / |
| Vitamin B9 | 5-methyltetrahydro | 400 µg | 100 | / | / |
| folate (Metafolin®) | |||||
| Folic acid | / | / | 200 µg | 100 | |
| Biotin | Biotin | 100 µg | 200 | 150 µg | 300 |
| Iron | 14 mg | 100 | 14 mg | 100 | |
| Chromium | 80 µg | 200 | / | / | |
| Copper | 1 mg | 100 | 1 mg | 100 | |
| Iodine | 150 µg | 100 | 50 µg | 33 | |
| Manganese | 1 mg | 50 | 3.5 mg | 175 | |
| Magnesium | 60 mg | 16 | 100 mg | 27 | |
| Molybdenum | 100 µg | 200 | 75 µg | 150 | |
| Selenium | 100 µg | 182 | 35 µg | 64 | |
| Zinc | 22.5 mg | 225 | 15 mg | 150 | |
| Choline | Choline bitartrate | 5 mg | / | / | / |
| Inositol | 5 mg | / | / | / |
Table 1: Dosages of supplement ingredients.
Blood serum vitamin D values lower than 20 μg/L were treated in both groups with D-cure®, 1ml ampoules containing 25000 IU or 625 μg vitamin D once a week or every two weeks.
Data collection and follow-up
Patients were seen during four different ambulatory visits: 4 to 2 weeks before surgery (baseline) and 4, 7 and 12 months after surgery. During these visits, standard laboratory tests were performed including total, HDL, non-HDL and LDL cholesterol, glucose, triglycerides, calcium, iron, transferrin, transferrin saturation, ferritin, vitamin B12, folic acid and vitamin D. During the post-surgery visits, treatment compliance was emphasized by a clinical pharmacist. For the analysis of the supplementation impact, each value was compared with baseline.
Analysis and sample size calculation
A per protocol analysis is used to measure the effect of the multivitamin preparation, excluding patients who received additional medication because of a nutritional deficiency during the study. The sample size was calculated with G*power version 3.1 and was based on the modification of the vitamin B12 levels after surgery in relation to the baseline values. To detect an effect of 0.8 with 90% power and 0.05% significance, a minimum of 34 patients was required per group.
Continuous data were summarized by their mean, standard deviation, median, minimum and maximum. The effect of treatment (Specialized vs Standard treatment) on changes in blood levels during follow-up in comparison with baseline was studied in detail using generalized linear mixed-effects model.
Systematic deviations from the normality assumptions were verified using QQ-plots. Scatterplots of the residuals versus the predictions were used to check for systematic departures from the mean model. A value of p<0.05 was considered statistically significant. All analyses were performed in R version 3.2.0. (R Core Team, 2018).
Results
Population
In total, 159 patients were included of which 14 dropped out. 91 subjects were recruited in the intervention group and 54 in the control group.
Reasons for drop-outs were: originally planned for a gastric bypass, but underwent a sleeve gastrectomy (N=8), postponed surgery due to cardiovascular load and non-compliance to the smoking ban (N=1), postponed surgery because of cardiovascular load and too high levels of HbA1c (12%) (N=1), surgery cancelled by psychologist or dietitian (e.g. because of alcoholism, mental status) (N=2) and lost to follow-up (N=2).
At baseline, both groups were similar in terms of gender, age (see Table 2), pre-operative BMI, baseline values of all analyzed components, socio-economic status, civil state, profession and smoking status.
| Variable | All (N=145) | Intervention group (N=91) | Control group (N=54) |
|---|---|---|---|
| Gender | |||
| Male % (n/N) | 24.1 (35/145) | 26.4 (24/91) | 20.4 (11/54) |
| Female % (n/N) | 75.9 (110/145) | 73.6 (67/91) | 79.6 (43/54) |
| Age (Years) | |||
| Mean (SD) | 43.3 (11.7) | 43.7 (12.5) | 42.8 (10.4) |
| Min-Med-Max | 18-44-69 | 18-44-69 | 20-42.5-65 |
Table 2: Baseline characteristics of study population.
Due to the high number of drop-outs at 12 months follow-up, data could only be analyzed till 7 months after surgery.
Vitamin B12
In both groups, patients had on average the same baseline values (p=0.828). After 4 and 7 months, the intervention group showed significant higher serum vitamin B12 concentrations (p<0.001) in comparison with the control group.
The concentration of vitamin B12 is significantly higher at 4 months (p<0.001) and stays stable at 7 months. For the control group, a significant reduction of B12 concentrations is seen over time (p<0.001). The intervention group starts at a mean baseline B12 level of 342 ± 142.7 ng/L (n=82) and rises to 416 ± 172.6 ng/L (n=75) at 4 months and 421.0 ± 170.7 ng/L (n=44) at 7 months. On the other hand, the control group had mean baseline values of 349.4 ± 125.8 ng/L (n=47) and declines to 305.1 ± 100 ng/L (n=48) at 4 months and 261.4 ± 79 (n=39) at 7 months, see Figure 1. Compared with the reference values (197-771 ng/L), the values of the control group are still within the normal range but close to the lower reference limit.
Vitamin D
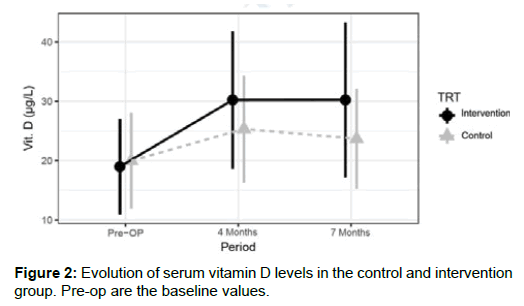
Baseline values for vitamin D were on average the same in both treatment groups (p=0.762). The analysis indicates that subjects following standard treatment had significant (p<0.001) lower mean vitamin D levels at 4 months and 7 months when compared to the intervention group. The intervention group showed on average higher vitamin D levels at all post-operative periods when compared to baseline (p<0.001). Vitamin D levels for the standard group started at 20.0 ± 8.0 μg/L (n=44) and evolved to 25.3 ± 9.0 μg/L (n=40) at 4 months and 23.7 ± 8.4 μg/L (n=34) at 7 months. The intervention group started at 19.0 ± 8.0 μg/L (n=79) and evolved to 30.2 ± 11.5 μg/L (n=65) at 4 months and 30.2 ± 13.0 μg/L (n=38) at 7 months, see Figure 2. The control group falls within the deficient reference values (10-24 μg/L) while the intervention group falls within the optimal vitamin D reference values (25-80 μg/L) at 7 months.
The extra vitamin D substitution under the form of D-cure® in case of severe vitamin D deficiency in both groups, also needs to be taken into account. Table 3 gives an overview of the starting moments of the administration of D-cure®. After 4 months, a significant higher number of patients started taking D-cure® in comparison with the intervention group. Also at 7 months, a non-significant but still double amount of patients were taking extra vitamin D in the control group.
| Start D-cure® (number) | Control group | Intervention group | Difference with 95% CI, P- value |
|---|---|---|---|
| At 4 months | 18/40 or 45% | 6/65 or 9% | 35.7% (16.8%; 54.7%), p<0.001 |
| At 7 months | 9/34 or 26% | 4/38 or 11% | 15.9% (-4.6%; 36.5%), p=0.147 |
Table 3: D-cure® statistics.
Iron and ferritin
Both groups have a similar mean baseline Iron value and show a non-significant small rise at 7 months (p=0.074). For ferritin, no significant evidence of treatment effect (p=0.238) or change over time (p=0.058) is observed. Both iron and ferritin values fall within the normal ranges of 50 μg/dL-170 μg/dL and 17 μg/L-291 μg/L respectively (Table 4).
| Pre-OP | 4 Months | 7 Months | |
|---|---|---|---|
| Iron (µg/dL) | |||
| Intervention | 82.9 ± 30.9 (n=82) | 76.6 ± 23.3 (n=68) | 90.2 ± 30.2 (n=38) |
| Standard | 87.5 ± 31.3 (n=47) | 86.4 ± 29.7 (n=38) | 96.3 ± 27.6 (n=32) |
| Ferritin (µg/dL) | |||
| Intervention | 109.6 ± 85.6 (n= 83) | 115.3 ± 110.4 (n=69) | 96.4 ± 102.4 (n=42) |
| Standard | 95.5 ± 103.1 (n=45) | 100.6 ± 95.6 (n=36) | 90.9 ± 90.5 (n=30) |
Table 4: Iron and ferritin levels.
Since a lot of data is missing for transferrin and transferrin saturation, the results aren’t shown. The iron status is very variable and an individual characteristic depending mostly of gender and age. Supplementing higher amounts than necessary, leading to an excess of iron, is not recommended since an excess of iron works pro-oxidative due to the conversion of superoxide anion (O2¯) and hydrogen peroxide (H2O2) into the extremely reactive hydroxyl radical (˙OH) via the Haber-Weiss reaction [29]. It’s recommended to supplement iron based on individual needs (in case of low ferritin) to avoid this pro-oxidative effect.
Calcium
Serum calcium concentration was analyzed in both groups. With the constant homeostasis between calcium in blood serum, bones and it’s excretion, serum calcium levels will not reflect the level of calcium depletion in the body. A decline in serum calcium will be resolved by the release of calcium from the bones. If serum calcium concentrations rise again, the bones will resorb the excess to restore calcium stores maintaining the serum calcium balance [30]. A combination of serum calcium, PTH and vitamin D would be a better indication of calcium loss.
In the control group, calcium levels were stable at 2.3 ± 0.1 mmol/L. In the intervention group, the calcium levels were 2.4 ± 0.1 mmol/L at all timepoints indicating the calcium homeostasis.
Folic acid
There was no evidence of a significant treatment effect for folic acid values (p=0.391). Folic acid values were on average higher postsurgery when compared to baseline and always within the normal ranges (3.9 μg/L-26.8 μg/L) (see Table 5). Blood serum folic acid values for the control group were 8.5 ± 5.2 μg/L (n=48) before surgery, 9.9 ± 4.7 μg/L (n=41) 4 months and 10.3 ± 4.5 μg/L (n=36) 7 months after surgery. For the intervention group, baseline values were 6.7 ± 4.1 μg/L (n=80), at 4 months 10 ± 5.1 μg/L (n=68) and at 7 months 10.3 ± 4.5 μg/L (n=36).
| 4 Months | 7 Months | 12 Months | |
|---|---|---|---|
| Mean change from baseline | (95% CI) 2.42 (1.43; 3.41) | 2.74 (1.62; 3.86) | 3.16 (1.72; 4.60) |
| p-value Period | <0.001 | <0.001 | <0.001 |
Table 5: General mean folic acid changes from baseline.
Glucose and triglycerides
Our patient population underwent the restrictive RYGB resulting in a lower food, glucose and fat intake. Our data confirmed this theory as mean glucose levels significantly reduce over time in both groups (p<0.001) and mean triglyceride levels decline at 4 months (p=0.071) and 7 months (p=0.002), see Table 6.
| Pre-OP | 4 Months | 7 Months | |
|---|---|---|---|
| Glucose (mg/dL) | |||
| Intervention | 109.9 ± 26.6 (n=85) | 91 ± 28.3 (n=69) | 84.6 ± 9.8 (n=44) |
| Standard | 102.7 ± 22.3 (n=48) | 89.3 ± 24.1 (n=45) | 83.5 ± 8.4 (n=39) |
| Triglycerides (mg/dL) | |||
| Intervention | 121.2 ± 65.7 (n=85) | 110.0 ± 44.8 (n=67) | 100.1 ± 41.2 (n=39) |
| Standard | 123.9 ± 72 (n=49) | 117.3 ± 49.4 (n=47) | 103.1 ± 50.9 (n=39) |
Table 6: Glucose and triglyceride levels.
Cholesterol
Overall, a significant reduction in mean total, LDL and non-HDL cholesterol was observed at 4 and 7 months (p<0.001 for both periods) compared with baseline (Table 6). There was no significant treatment effect. For HDL, the baseline values were on average the same for both treatment groups (p=0.206). In general, a decline of the values at 4 months and a slow rise at 7 months is observed (Table 7).
| Pre-OP | 4 Months | 7 Months | |
|---|---|---|---|
| Total Cholesterol (mg/dL) | |||
| Intervention | 200.9 ± 40.8 (n=87) | 155.5 ± 34.8 (n=70) | 171.6 ± 29.8 (n=41) |
| Standard | 199.8 ± 35.5 (n=49) | 162.7 ± 33.1 (n=47) | 164.3 ± 36.5 (n=39) |
| HDL (mg/dL) | |||
| Intervention | 52.8 ± 15.2 (n=85) | 45.8 ± 10.1 (n=65) | 57.2 ± 13.2 (n=37) |
| Standard | 56.1 ± 16.0 (n=50) | 46.2 ± 12.4 (n=45) | 52 ± 12.9 (n=38) |
| Non-HDL (mg/dL) | |||
| Intervention | 147.2 ± 36.7 (n=83) | 110.6 ± 28.9 (n=62) | 112.4 ± 28.4 (n=38) |
| Standard | 141.2 ± 28.1 (n=33) | 121.8 ± 62.4 (n=44) | 110.3 ± 31.7 (n=38) |
| LDL (mg/dL) | |||
| Intervention | 123.1 ± 35.4 (n=85) | 90.4 ± 27.4 (n=67) | 96 ± 25.5 (n=37) |
| Standard | 119.2 ± 31.8 (n=50) | 95.5 ± 27.2 (n=46) | 92.4 ± 29.6 (n=38) |
Table 7: Total, HDL, non-HDL and LDL cholesterol levels.
Discussion
This study confirms the need for supplementation with an adapted treatment using a specialized multivitamin after Roux-en-Y gastric bypass, which has also been confirmed in other studies like Perin et al. [31,32]. A standard multivitamin was associated with a substantial decline in vitamin B12 and vitamin D concentrations over 7 months while these values rise and stabilize with the intake of a specialized multivitamin. This effect is due to the adapted amounts and composition with bioavailable and bioactive forms of vitamins and minerals as it contains e.g. 500x more vitamin B12 and 10x more vitamin D in comparison with the standard multivitamin. The decline in blood sugar, triglyceride and cholesterol levels are due to the food restriction and weight loss. As calcium is a stable blood parameter, no effect could be confirmed. Nevertheless supplementation of calcium shouldn’t be neglected as an increased facture risk after bariatric surgery at the long term has been confirmed [13,33].
Vitamin B12
After a surgical intervention with a malabsorptive character, such as RYGB, an average of 1 patient out of 3 has a shortage of cobalamin or vitamin B12 [34-36]. A prevalence of 10% up to 75% is reported after gastric bypass [36-39]. This deficiency can already be observed after six months, but the majority of the deficiencies appear due to depletion of the liver, about one year after surgery [37]. The most important factor is the decreased production of Intrinsic Factor (IF). Due to a reduced presence of parietal cells after bypass, IF is less produced, less vitamin B12-IF complex formed and consequently less vitamin B12 absorbed. Additionally, the decreased intake and bad tolerance of red meat but also the reduced gastric acid production play an extra role [34,35,40]. This vitamin plays an important role in the production of red blood cells. A deficiency can be presented as macrocytic anemia or even in the long run, as an irreversible neuropathy [34-36]. Frequent monitoring and adequate preventive substitution are therefore recommended [18,19,27]. In case of deficiency, higher doses such as 1000 μg orally or 1000 μg intramuscular vitamin B12 are recommended for a certain period of time [18,19,27,34]. As intramuscular injections are very painful, oral substitution is recommended and higher dosages of vitamin B12 are found in specialized multivitamins.
The observations described above have been confirmed by this study as a significant decline of vitamin B12 is observed in the control group with a drop from 349.4 ± 125.8 to 261.4 ± 79 ng/L after 7 months. With a dose 500 times higher than the standard multivitamin, a rise and stabilization of vitamin B12 could be established 7 months after surgery with the specialized multivitamin (from 342.0 ± 142.7 to 421.0 ± 170.7). In literature and several large community studies, reflection points for vitamin B12 usually fall between 200 pmol/l and 500 pmol/l, which is approximately 271-677 ng/L [41]. Vogiatzoglou et al. defined a vitamin B12 “repleted” group as ≥ 400 pmol/l which was also used by Selhub et al. [42,43]. As the control group falls below above mentioned ranges after 7 months, they can be seen as subclinical cobalamin deficient, defined by carmel as state of ‘mild metabolic abnormalities without clinical signs or symptoms [41,44]. Despite not having symptoms, this group needs follow-up and should consider extra supplementation as other pathologies could develop unnoticed such as neuropathy [41].
A similar trial observed 7.9% deficiencies after one year with the daily supplementation of 12.5μg vitamin B12, while Navarro et al. reported 10% deficiencies with the intake of 6 μg vitamin B12 [31,45]. Because most deficiencies appear about one year after surgery [37], the adapted vitamin supplement has been reconfirmed. To determine the amount needed to maintain adequate vitamin B12 levels, it can be argued that, Dogan et al. observed still 1,6% deficiencies after 12 months taking 350 μg vitamin B12 per day [31]. With a daily dose of 500 μg in this study, no deficiencies nor side effects were observed. The European Food Safety Authority (EFSA) confirms that vitamin B12 is completely safe, even after excessive intake in the long term. As cobalamin is not considered as carcinogen or genotoxic, no tolerable upper intake level is recorded [46,47].
Vitamin D
Vitamin D is a lipo-soluble vitamin for which in literature at least 60%-65% of the general population has a pre-operative deficiency (<20 ng/mL). If 30 ng/mL is taken as cut-off, the percentage increases up to 85%-90%, which is not surprising as even the majority of the European population is not reaching this limit [48]. As vitamin D is sequestered in the adipose tissue, it’s bioavailability decreases with an increasing BMI leading to huge shortages in the bariatric population with even pre-operative supplementation [39,49,50]. After surgery, a daily intake of 3000 units (IU) per day and a routine screening are recommended by the American Association of Clinical Endocrinologists (AACE) [18,32,51]. Deficiencies on the long term are very harmful and could lead to secondary hyperparathyroidism and decreased bone density with risk of osteoporosis and fractures [13,33]. 3000 IU were administered as a combination of 2000 IU in the specialized multivitamin and 1000 IU daily vitamin D in the calcium supplement. This combination leads to a stabilization of vitamin D levels at 30.2 ± 13.0 μg/L while the control group ended up at 23.7 ± 8.4 μg/L 7 months post-surgery. Moreover, the control group also received significantly more D-cure® than the intervention group at 4 and 7 months post-surgery (p<0,001 and p<0.147 respectively), confirming the high needs for vitamin D.
Calcium
Calcium is mainly absorbed in the duodenum and proximal jejunum. Moreover, the absorption of calcium carbonate decreases in absence of an acid environment while calcium citrate has an acid independent absorption [13,34,52]. Different guidelines and literature sources recommend a daily and life-long absorption of 1000-1200 mg of calcium as calcium citrate, which is supposed to be essential after a gastric bypass [18,19,26,27,34]. Calcium cannot be supplemented without vitamin D and vice versa because deficiencies of both elements play a role in the development of hypoparathyroidism and osteoporosis with a higher fracture risk [13,18,33]. There is evidence of a bone mineral density decrease after bariatric surgery and a higher risk of developing secondary hypoparathyroidism [53-55]. Since 2012, some cohort studies have been published analyzing an increased fracture risk after bariatric surgery at the long term (4- 12 year follow-up) [56-58]. These studies show an increased risk of fractures, typically osteoporotic fractures, after 4,4 to 12 year after surgery [53-55]. Probably due to the very small proportion of patients with a gastric bypass, this risk was not always significant.
Concerning the analysis of calcium in blood serum, no relevant differences after surgery between both groups were noticed in this trial (p>0,05). This doesn’t necessarily conclude that supplementation with calcium doesn’t have any utility as calcium levels remain stable until critically low availability in the body [30]. Also, low calcium intake only leads to visible effects such as brittle bones and fractures after a longer period of time. A more suitable marker for calcium metabolism is PTH which is elevated during low calcium intake [59].
Iron: The incidence of iron deficiency after RYGB has been reported from 15% to 60% due to a combination of various causes [21,35,60]. First of all, the poor tolerance for red meat leads to a reduced intake of iron by on average 50% after gastric bypass [21,61]. Secondly, the restricted gastric volume and the reduced HCl production are responsible for a limited release of iron from his protein matrix, but especially for the restricted transformation of the ferri-form into the reduced ferro-form (Iron 2+) which is the best absorbable form via the DMT1-transporter [61]. Last, the reduction of the absorption surface (duodenum and proximal jejunum) leads to the increased prevalence of iron deficiency [21,22]. Especially young fertile women and women with menorrhagia should be careful after RYGB due to the blood loss during the menses [19,35,36,62]. For these patients, it is recommended to give an additional iron supplement after a gastric bypass to avoid iron-deficiency anemia [19,35,36,62]. Most recommendations mention at least 45-60 mg of extra elementary iron per day via a multivitamin or a special supplement [18,19,27]. In some cases, intravenous administration of iron can still be necessary despite these preventive actions [63].
Even though in other studies, iron deficiency appears as one of the most frequent deficiencies after RYGB, no significant difference could be demonstrated in iron and ferritin levels between both groups. Only a general non-significant small rise can be observed over time (p=0.074). This could be due to the missing data regarding iron injections as a rise is expected in the intervention group due to extra iron supplementation in pre-menopausal women (<45 y). Also, an iron deficiency with anemia occurs principally only a few years after surgery [27,35]. Another reason might be the increased concentration of hepcidin, produced by the chronic inflammation in obesity which leads to the obstruction of oral iron absorption [64]. Thus it cannot be concluded that 42 mg of iron is sufficient or insufficient. On the other hand, iron supplementation is not recommended for patients with sufficient iron. Excessive iron can cause a pro-oxidative effect releasing free hydroxyl radicals due to the Haber-Weiss reaction [28,65]. Supplementation according to needs and deficiencies, seems to be the best solution in case of iron.
Glucose, triglyceride levels and cholesterol
General improvements in metabolic syndrome related risk factors like insulin resistance, glucose and fat intolerance (increased triglycerides, decreased HDL) are generally known after weight loss surgery [66]. These improvements in glycemic control and hypertriglyceridemia are due to the caloric restriction promoted by RYGB leading to a reduction of fat and sugar intake, a decrease in insulin resistance and a decrease in secretion of Very Low-Density Lipoprotein (VLDL) [66]. Also, the reduction of abdominal fat and decreased flux of free fatty acids to the liver leads to a decreased secretion of triglyceride-rich lipoproteins by the liver and a decline in cholesterol [67]. A decrease in glucose, triglyceride levels and improvement of cholesterol (decline in total cholesterol, Non-HDL and LDL) are seen in this trial, confirming the beneficial effects of a RYGB as also stated by Guilbert et al. and To VT et al. [68,69].
Active and organic salt forms: For vitamins, genetic polymorphisms in the general population are known to cause conversion problems to their active form. For example, 20% of the general population is restricted in the conversion of folic acid to 5-methyltetrahydrofolate (5-MTHF). Therefore, supplementation with the active form (5-MTHF) is recommended. Also for minerals, problems occur. As bariatric patients secrete less gastric acid, they might benefit from organic salt forms of minerals. These organic salt forms dissociate effectively in a less acidic environment, e.g. calcium citrate is better absorbed than calcium carbonate in the absence of an acid environment [35,53].
Limitations
Several limitations of the use of a retrospective control group should be considered. As no interrogation was possible, data were reconstructed by using electronic patient data, follow-up notes and electronic prescriptions. Due to this, there’s still a chance that the intake of some medications and/or supplements was not documented.
Another limitation of this study is the lack of information on (healthy) food. The nutritional intake could be very interesting to analyze next to the intake of vitamin and mineral supplements. Also, a bigger effect of a specialized supplement will be seen after a longer time period as adequate reserves in the human body may mask a lower in- and uptake of vitamins and minerals and the development of deficiencies in the short term.
Conclusion
In summary, a specialized supplement enriched in specific nutrients is superior to a standard multivitamin in adjusting for deficiencies after bariatric surgery, especially for vitamin B12 and vitamin D. Depending on the iron status, extra iron supplementation can be necessary next to the intake of multivitamins and calcium for life. The nutritional status should be monitored closely and consistently after bariatric surgery to avoid deficiencies.
References
- Buchwald H, Ikramuddin B, Dorman RB, Schone JL, Dixon JB (2011) Management of the metabolic/bariatric surgery patient. Am J Med 124: 1099-105.
- Aron-Wisnewsky J, Verger EO, Bounaix C, Dao MC, Oppert JM, et al. (2016) Nutritional and protein deficiencies in the short term following both gastric bypass and gastric banding. PLoS One 11: e0149588.
- Andreu A, Moize V, Rodríguez L, Flores L, Vidal J (2010) Protein intake, body composition, and protein status following bariatric surgery. Obesity Surg 20: 1509-1515.
- Miller AD, Smith KM (2006) Medication and nutrient administration considerations after bariatric surgery. Am J Health Syst Pharm 63: 1852-1857.
- Giusti V, Theytaz F, Di Vetta V, Clarisse M, Suter M, et al. (2016) Energy and macronutrient intake after gastric bypass for morbid obesity: A 3-y observational study focused on protein consumption. Am J Clin Nutr 103: 18-24.
- Ito MK, Goncalves VS, Faria SL, Moize V, Porporatti AL, et al. (2017) Effect of protein intake on the protein status and lean mass of post-bariatric surgery patients: A systematic review. Obes Surg 27: 502-512.
- Schollenberger AE, Karschin J, Meile T, Kuper MA, Konigsrainer A, et al. (2016) Impact of protein supplementation after bariatric surgery: A randomized controlled double-blind pilot study. Nutrition 32: 186-192.
- Lopes Gomes D, Moehlecke M, Lopes da Silva F, Dutra E, D'Agord Schaan B, et al. (2017) Whey protein supplementation enhances body fat and weight loss in women long after bariatric surgery: A randomized controlled trial. Obes Surg 27: 424-431.
- Verger EO, Aron-Wisnewsky J, Dao MC, Kayser BD, Oppert JM, et al. (2016) Micronutrient and protein deficiencies after gastric bypass and sleeve gastrectomy: A 1-year follow-up. Obes Surg 26: 785-796.
- Gehrer S, Kern B, Peters T, Christoffel-Courtin C, Peterli R (2010) Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB) a prospective study. Obes Surg 20: 447-453.
- Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ (2008) Nutritional deficiencies in morbidly obese patients: A new form of malnutrition? Obes Surg 18: 870-876.
- Aarts EO, Van Wageningen B, Janssen IM, Berends FJ (2012) Prevalence of anemia and related deficiencies in the first year following laparoscopic gastric bypass for morbid obesity. J Obesity 2012: 191-198.
- Slater GH, Ren CJ, Siegel N, Williams T, Barr D, et al. (2004) Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg 8: 48-55.
- Sauerland S, Angrisani L, Belachew M, Chevallier JM, Favretti F, et al. (2005) Obesity surgery: Evidence-based guidelines of the european association for endoscopic surgery (EAES). Surg Endosc 19: 200-221.
- Donadelli SP, Junqueira-Franco MV, Mattos Donadelli CA, Salgado WJ, Ceneviva R, et al. (2012) Daily vitamin supplementation and hypovitaminosis after obesity surgery. Nutrition 28: 391-396.
- Alvarez-Leite JI (2004) Nutrient deficiencies secondary to bariatric surgery. Cur Opin Clin Nutr Metab Care 7: 569-575.
- Stein J, Stier C, Raab H, Weiner (2014) Review article: The nutritional and pharmacological consequences of obesity surgery. Aliment Pharmacol Ther 40: 582-609.
- Mechanic JI, Youdin A, Jones DB, Garvey WT, Hurley DL, et al. (2013) Clinical practice guidelines for the perioperative nutritional, metabolic and nonsurgical support of the bariatric surgery patient 2013 update: Cosponsored by Am Association of Clinical Endocrinologists, and American society for Metabolic and bariatric surgery. Obesity 21: S1-S27.
- Heber D, Greenway FL, Kaplan LM, Livingston J (2010) Endocrine and nutritional management of the post-bariatric surgery patient: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 95: 4823-4843.
- Ducloux R, Nobecourt R, Chevalier JM, Ducloux H, Altman JJ (2011) Vitamin D deficiency before bariatric surgery: should supplement intake be routinely prescribed? Obes Surg 21: 556-560.
- Kushner RF (2006) Micronutrient deficiencies and bariatric surgery. Cur Opin Endocrinol Diab 13: 405-411.
- Davies DJ, Baxter JD, Baxter JN (2007) Nutritional deficiencies after bariatric surgery. Obes Surg 17: 1150-1158.
- Shanker P, Boylan M, Sriram K (2010) Micronutrient deficiencies after bariatric surgery. Nutrition 26: 1031-1037.
- Landais A (2014) Neurological complications of bariatric surgery. Obes Surg 24: 1800-1807.
- Kailasam VK, DeCastro C, Macaluso C, Kleiman A (2015) Postbariatric surgery neuropathic pain (PBSNP): Case report, literature review, and treatment options. Pain Med 16: 374-382.
- Valentino D, Sriram K, Shanker P (2011) Update on micronutrients in bariatric surgery. Curr Opin Clin Nutr Metab Care 14: 635-641.
- Ziegler O, Sirveaux MA, Brunaud L, Reibel O, Quilliot R (2009) Medical follow-up after bariatric surgery: nutritional and drug issues. General recommendations for the prevention and treatment of nutritional deficiencies. Diabetes Metab 35: 544-557.
- Patel JS, Mundi MS, Hurt RT, Wolfe B, Martindale RG (2017) Micronutrient deficiencies after bariatric surgery: an emphasis on vitamins and trace mineral. Nutrition Clin Practice 32: 471-480.
- Puntarulo S (2005) Iron, oxidative stress and human health. Mol Aspects Med 26: 299-312.
- Peacock M (2010) Calcium metabolism in health and disease. Clin J Am Soc Nephrol 5: S23-S30.
- Dogan K, Aarts EO, Koehestanie P, Betzel B, Ploeger N, et al. (2014) Optimization of vitamin suppletion after Roux-en-Y Gastric bypass surgery can lower postoperative deficiencies: A randomized controlled trial. Medicine 93: 25.
- Perin J, Prokopowicz G, Furtado M, Papas M, Steele KE (2018) A randomized trial of a novel chewable multivitamin and mineral supplement following Roux-en-Y gastric bypass. Obes Surg 28: 2406-2420.
- Bland CM, Quidley AM, Love AL, Yeager CL, McMichael B, et al. (2016) Boostaver, long-term pharmacotherapy considerations in the bariatric surgery patient. Amer J Heal System Pharm 73: 1230-1242.
- Sawaya RA, Jaffe J, Friedenberg J, Friedenberg FK (2012) Vitamin, mineral, and drug absorption following bariatric surgery. Curr Drug Metab 13: 1345-1355.
- Skroubis G, Sakellaropoulos G, Pouggouras K, Mead N, Nikiforidis G (2002) Comparison of nutritional deficiencies after Roux-En-Y gastric bypass and after biliopancreatic diversion with Roux-en-Y gastric bypass. Obes Surg 12: 551-558.
- Rhode BM, Arseneau P, Cooper BA, Katz M, Filfix BM, et al. (1996) Vitamin B12 deficiency after gastric surgery for obesity. Am J Clin Nutr 63: 103-109.
- Brolin RE, Gorman JH, Gorman RC, Petschenik AJ, Bradley LY, et al. (1998) Are vitamin B12 and folate deficiency clinically important after Roux-en-Y gastric bypass? J Gastrointest Surg 2: 436-442.
- MacLean LD, Rhode BM, Shizgal HM (1983) Nutrition following gastric operations for morbid obesity. Am Surg 198: 347-355.
- Boyland LM, Sugerman HJ, Driskell SA (1985) Vitamin E, vitamin B-6, vitamin B-12, and folate status of gastric bypass surgery patients. J Am Diet Assoc 88: 579-585
- Marcuard SP, Sinar DR, Swanson MS, Silverman JS, Levine JS (1989) Absence of luminal intrinsic factor after gastric bypass surgery for morbid obesity. Dig Dis Sci 34: 1238-1242.
- Smith AD, Refsum H (2009) Do we need to reconsider the desirable blood level of vitamin B12? J Intern Med 271: 179-182.
- Vogiatzoglou A, Oulhaj A, Smith AD, Nurk E, Drevon AE, et al. (2009) Determinants of plasma methylmalonic acid in a large population: implications for assessment of vitamin B12 status. Clin Chem 55: 2198-2206.
- Selhub J, Jacques PF, Dallal G, Choumenkovitch S, Rogers G (2007) The use of blood concentrations of vitamins and their respective functional indicators to define folate and vitamin B12 status. Food Nutr Bull 29: S67-73.
- Carmel R (2011) Biomarkers of cobalamin (Vitamin B12) status in the epidemiologic setting: a critical overview of context, applications, and performance characteristics of cobalamin, methylmalonic acid and holotranscobalamin II. Am J Clin Nutr 94: 348S-358S.
- Navarro M, Wood RJ (2003) Plasma changes in micronutrients following a multivitamin and mineral supplement in healthy adults. J Am Coll Nutr 22: 124-132.
- EFSA NDA Panel (2015) Scientific opinion on dietary reference values for cobalamin (Vitamin B12), EFSA Journal 13: 4150.
- Scientific Committee on Food (2000) Opinion of the scientific committee on food on the tolerable upper intake level of Folate. European Commission 1-42.
- Cashman KD, Dowling KZ, Skrabakova Z, Gonzalez-Gross M, Valtuena V, et al. (2016) Vitamin D deficiency in Europe: Pandemic? Am J Clin Nutr 103: 1033-1044.
- Kimmons JE, Blanck HM, Tohill BC, Zhang TE, Khan LK (2006) Associations between body mass index and the prevalence of low micronutrient levels among US adults. Med Gen Med 8: 59
- Lespessailles E, Toumi H (2017) Vitamin D alteration associated with obesity and bariatric surgery. Exp Biol Med 242: 1086-1094.
- Holick MF, Binkley NC, Bischoff-Ferrari BC, Gordon MC, Hanley DA, et al. (2007) Evaluation, treatment, and prevention of vitamin D Deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 96: 1911-1930.
- Sakhaee T, Bhuket B, Adams-Huet A, Rao DS (1999) Meta-analysis of calcium bioavailability: A comparison of calcium citrate with calcium carbonate. Am J Ther 6: 313-321.
- Costa TM, Paganoto M, Radominski RB, Borba VZ (2016) Impact of deficient nutrition in bone mass after bariatric surgery. Arg Bras Cir Dig 29: 38-42.
- Pizzorno L (2016) Bariatric surgery: Bad to the bone, part 1. Integr Med (Encinitas) 15: 48-54.
- Pizzorno L (2016) Bariatric surgery: Bad to the bone, part 2, Integr Med (Encinitas) 15: 35-46.
- Nakamura KM, Haglind EG, Clowes JN, Achenbach AS, Atkinson EJ, et al. (2014) Fracture risk following bariatric surgery: a population-based study. Osteoporos Int 25: 151-158.
- Lu CW, Chang YK, Chang HH, Kuo CK, Huang CT, et al. (2015) Fracture risk after bariatric surgery: A 12-year nationwide cohort study. Medicine 94: 48.
- Rousseau C, Jean S, Gamache P, Lebel S, Mac-Way F, et al. (2016) Change in fracture risk and fracture pattern after bariatric surgery: Nested case-control study. BMJ 3794.
- Walker HK, Hall WD, Hurst JW (1990) Clinical methods: The history, physical and laboratory examinations, (3rd ed). Chest 143: e121s-e141s.
- Poitou BC, Ciangura C, Coupaye M, Czernichow CS, Bouillot JL (2007) Nutritional deficiency after gastric bypass: diagnosis, prevention and treatment. Diabetes Metab 33: 13-24.
- Ruz M, Carrasco F, Rojas P, Codeceo J, Inostroza J, et al. (2009) Iron absorption and iron status are reduced after roux-en-y gastric bypass. Am J Clin Nutr 90: 527-532.
- Brolin RE, Gorman GE, Gorman RC, Petschenik AJ, Bradley AR, et al. (2010) Prophylactic iron supplementation after Roux-en-Y gastric bypass: a prospective, double- blind, randomized study. Arch Surg 133: 740-744.
- Bal B, Koch TR, Finelli FC, Sarr MG (2010) Managing medical and surgical disorders after divided Roux-en-Y gastric bypass surgery. Nat Rev Gastroenterol Hepatol 7: 320-334.
- Gesquiere I, Lannoo M, Augustijns P, Matthys C, Van der Schueren B, et al. (2014) Iron deficiency after Roux-en-Y gastric bypass: Insufficient iron absorption from oral iron supplements. Obes Surg 24: 56-61.
- Wiegand HL, Orths CT, Kerpen K, Lutze HZ, Schmidt TC (2017) Investigation of the iron- peroxo complex in the fenton reaction: kinetic indication, decay kinetics, and hydroxyl radical yields. Environ 51: 14321-14329.
- Junges VM, Cavalheiro JM, Fam EF, Closs VE, Moraes JF, et al. (2017) Gottlieb, impact of roux-en-y gastric bypass surgery on metabolic syndrome components and on the use of associated drugs in obese patients. Arg Gastroenterol 54: 139-144.
- Elhag W, El Ansari W, Abdulrazzaq S, Abdullah S, Elsherif A (2018) Evolution of 29 anthropometric, nutritional, and cardiometabolic paameters among morbidly obese adolescents 2 years post sleeve gastrectomy. Obes Surg 28: 474-482.
- To VT, Huttl TP, Lang R, Piotrowski K, Parhofer KG (2012) Changes in Body weight, glucose homeostasis, lipid profiles and metabolic syndrome after restrictive bariatric surgery. Exp Clin Endocrinol Diab 120: 547-552.
- Guilbert L, Ortiz CJ, Espinosa O, Sepulveda EJ, Pina T, et al. (2018) Metabolic syndrome 2 years after laparoscopic gastric bypass. Int J Surg 52: 264-268.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi