Research Article, Dent Health Curr Res Vol: 5 Issue: 1
The Effect of Silver Oxide Nanoparticles on the Antibacterial Property and Shear Bond Strength of Dental Composite
Suraj Naslapur, Sameer Ahmad Malik*, Laxmikanth SM and Ramachandra CS
Department Orthodontics and Dentofacial Orthopedics, AECS Maaruti College of Dental Sciences and Research Centre, Bangalore, India
*Corresponding Author : Sameer Ahmad Malik
Post Graduate MDS student, Department of Orthodontics and Dentofacial Orthdopedics AECS Maaruti College of Dental Sciences and Research Centre, off Bannerghatta road, Bangalore 560076, India
Tel: (+91) 8553340994
E-mail: malik11sameer@gmail.com
Received: December 10, 2018 Accepted: January 05, 2019 Published: January 14, 2019
Citation: Naslapur S, Malik SA, Laxmikanth SM, Ramachandra CS (2019) The Effect of Silver Oxide Nanoparticles on the Antibacterial Property and Shear Bond Strength of Dental Composite. Dent Health Curr Res 5:1. doi: 10.4172/2470-0886.1000138
Abstract
Background: Although the mechanical properties of composite resin for orthodontic bonding have been improved substantially, their antibacterial properties are still limited. Various researchers have attempted to reduce plaque accumulation on composite adhesive material by incorporation of bactericidal agents. Silver has a significant antimicrobial activity and is effective against streptococci of the human oral cavity so it might be useful as an antibacterial agent incorporated into composite resin especially when applied in nanometer sizes. Thus the aim of this study is to evaluate if the addition of silver oxide nanoparticles at various proportions with orthodontic bonding resin as to influence their antibacterial properties and to evaluate if the addition of silver oxide nanoparticles at various proportions with orthodontic bonding resin will affect their shear bond strength.
Methodology: This study was done on one control group and two experimental groups. The control group consisted of Transbond XT and the two experimental group consisted of Transbond XT with 5% Silver oxide Nanoparticles and Transbond XT with 10% Silver oxide Nanoparticles respectively. Each group containing 10 specimens was subjected to antibacterial activity tested on Streptococcus mutans. For the antibacterial testing disk diffusion assay method was used. 30 Composite discs were made using molds each group containing 10 Composite discs. Similarly each group containing 10 specimens was subjected to shear bond strength testing using Instron universal testing machine For shear bond strength testing 30 tooth were bonding, 10 tooth in each group and were assessed for shear bond strength. The results were analyzed using one way ANOVA test and Pearson comparison.
Results: A statistically significant difference is found in the antibacterial activity and shear bond strength of the samples compared. The group containing Transbond XT with 10% Silver oxide Nanoparticles was found to have the highest antibacterial activity followed by group containing Transbond XT with 5% Silver oxide Nanoparticles and the least with the control group which contained only Transbond XT. The results for the shear bond strength showed that incorporation of silver oxide nanoparticles decreases the shear bond strength of composite resin. Incorporation of silver oxide nanoparticles containing 5% of silver reduces the bond strength to a level comparable to the control group. Further increasing the amount of silver nanoparticles to the 10% level demonstrates a decline in the shear bond strength.
Conclusion: Within the limitations of the present study, it can be concluded that the addition of Silver oxide Nanoparticles to Transbond XT composite significantly inhibits the growth of Streptococcus mutans. Whereas increasing the amount of particles to 10% has an undesirable effect on shear bond strength when compared to the control group.
Keywords: Silver oxide Nanoparticle, Streptococcus mutans, Shear bond strength
Introduction
Decades since their introduction into the field of orthodontics, composite resin adhesives undoubtedly remain the first choice of most orthodontists for bonding brackets [1]. Resin composites are widely used in dental clinics for the replacement of hard tissues. Although the mechanical properties and wear resistance of these materials have been improved substantially, their antibacterial properties are still of great interest [2]. Demineralization followed by white spot formation is a well-known complication in orthodontic therapy when fixed orthodontic appliances are used. Formation of incipient caries, commonly called white spot lesions (WSLs), is an unaesthetic, common side effect of orthodontic treatment. It is caused by increased numbers of Streptococcus mutans and other pathological microbes in the biofilm, decreased pH and compromised oral hygiene [3]. New orthodontic cements, adhesive resins, and hybrid cement-resin combinations offer improved physical properties and clinical benefits, but there are clear differences in the clinical indications and contraindications for each class of material. With an understanding of the features, benefits, and limitations, the practitioner can choose the material wisely to obtain optimal results [4]. For this purpose, various antimicrobial agents have been incorporated into orthodontic products and approved for intraoral use. Fluoride and chlorhexidine are the most common preventive additives for orthodontic use. Although initially strong, the released amounts of fluoride and chlorhexidine do not last for longer periods. In addition, when fluoride and chlorhexidine are used, antibacterial adhesives have a higher bond failure rate because incorporation of antibacterial agents into bonding adhesives affects their mechanical properties [5].
With the emergence of nanotechnology and the different behavior expressed by nanoparticles, attempts have been made to take advantage of this concept in orthodontic bonding [1]. The metallic nanoparticles are the most promising as they show good antibacterial properties due to their large surface area to volume ratios, which draw growing interest from researchers due to increasing microbial resistance against metal ions, antibiotics and the development of resistant strains. Nanotechnology discloses the use of elemental nanoparticles as active antibacterial ingredient for dental materials [6].
Silver has a long history of use in medicine as an antimicrobial agent. Resin composites containing silver ion- implanted fillers that release silver ions have been found to have antibacterial effects on oral streptococci. Several studies have demonstrated that composite incorporating silver-supported antibacterial material may be clinically useful due to its inhibitory effect against S. mutans and favorable mechanical properties. Recently, dental nanofilled resin composites were introduced in the market. The advantages of nanocomposite materials include excellent optical properties, easy handling characteristics, and superior polishiability. In addition, nanofillers can decrease surface roughness (SR) of orthodontic adhesives, which is one of the most significant factors for bacterial adhesion. To be accepted clinically, the new adhesives must provide superior antimicrobial activity and display comparable physical properties when compared with conventional adhesives [7]. Thus the aim of the study is to evaluate the anti-bacterial property and shear bond strength of silver oxide nanoparticles incorporated in the orthodontic bonding agents.
Methodology
This study consisted of 1 control group and 2 experimental groups:
Group 1: Control group: Transbond XT
Group 2: Experimental group: Transbond XT with 5 wt% AgO nanoparticle
Group 3: Experimental group: Transbond XT with 10 wt% AgO nanoparticles.
Preparation of sample for antibacterial testing
5% and 10% (by weight) of Silver Oxide Nanoparticles was used for sample preparation. This was measured by using a Digital weighing machine. For the first sample 100 mg of Silver Nano oxide particles was added to 2000 mg of Transbond XT using a Teflon coated spatula on 3 M mixing trays. For the second sample 200 mg of Silver Nano oxide particles was added to 2000 mg of Transbond XT using a Teflon coated spatula on 3 M mixing trays.
To measure the anti-bacterial activity: Each group containing 10 specimens was subjected to antibacterial activity tested on Streptococcus mutans. Disk diffusion assay method was used for the antibacterial activity testing. Brain heart infusion agar plates were inoculated with a 200 μL of bacterial solution (Streptococcus mutans) uniformly by a sterile swap. Composite discs were placed 2 cm apart on BHI agar plates which were inoculated with Streptococcus mutans. After 48 hour incubation, at 37°C, the zone of inhibition was measured with a digital calliper.
Preparation of composite discs incorporated with silver oxide nanoparticles
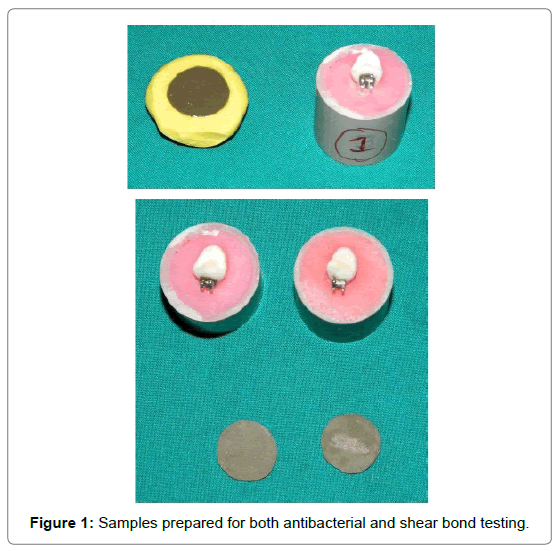
The nanoparticles with average size of 50 nm were added mechanically to composite (Transbond XT 3 M) at 5% and 10% by weight. The nanoparticles were mixed with the composite resin by a Teflon coated spatula continuously in a dark room for about 15 min until a homogenous mixture was obtained. Disc shaped molds were prepared with Vinyl Polysiloxane impression material (Zermack) of 10 mm in diameter and 2 mm in height. The composite was inserted into the mold and was covered with glass slides from top. The specimen was light polymerized for 2 min from both sides using an LED light curing unit. The samples of each study group were made by the same method as described above in the (VPS) mold. Transbond XT composite resin without Nano silver addition was used as Control group.
Preparation of sample for shear bond strength testing
Thirty specimens of extracted premolar were divided into 3 groups of 10 teeth each. The criteria for tooth selection included intact buccal enamel, not subjected to any pretreatment chemical agents such as hydrogen peroxide, Devoid of any developmental defects, Caries free, No cracks due to extraction forceps [8].
To measure the shear bond strength
The selected teeth were mounted in cold cure acrylic poured in hard plastic rings. The teeth were embedded in the acrylic vertically in such a way that the long axes of the teeth were parallel to the central axis of the hard plastic ring and were inserted in the ring till the cervical region of the tooth for maximum retention. The mounted blocks were divided into three groups of 10 samples each according to the addition of silver oxide nanoparticles. (0%, 5% and 10%)
Group 1: Control group--It consist of 10 ortho-organizer bracket bonded with Tranbond XT composite resin.
Group 2: Experimental group--It consist of 10 ortho-organizer bracket bonded with Tranbond XT composite resin containing 100 mg of Silver Nanoparticles
Group 3: Experimental group--It consist of 10 ortho-organizer bracket bonded with Tranbond XT composite resin containing 100 mg of Silver Nanoparticles.
The buccal surfaces of teeth were etched with 37% phosphoric acid solution for 60 sec. The teeth were then rinsed with a water spray for 30 sec and were dried with oil free air source for 20 sec. The buccal surfaces of the etched teeth appeared chalky white in color. A small amount of primer was applied to tooth surface and cured for 20 sec. The adhesive paste was applied to the bracket base and the brackets were positioned on the buccal surface of the teeth with the help of a bracket holding forceps and light cured for 40 sec and the adhesive was allowed to completely polymerize. The shear bond strength test was conducted in the laboratory of ARML lab, Bangalore. An Instron Machine (Model No.4467) was used in this study to record the shear bond strength. A crosshead speed of 0.2 mm/minute was used to debond the brackets. The plastic ring holding the tooth for shear test was positioned so that the force was applied to the bracket was parallel to the buccal surface of the tooth. For measuring the shear bond strength, the prepared plastic ring was fixed to the Aluminium Jig (Figures 1-5) which in turn was positioned in the lower cross head, with the long axis of the tooth and the buccal tube base parallel to the direction of force load applied. The cross head moved at a uniform speed of 0.2 mm/min. The load was progressively applied till the bracket got detached from the tooth surface and the reading was recorded in Megapascals (MPa) for every specimen.
Results
The present study was carried to measure the antibacterial activity and shear bond strength of silver oxide nanoparticles incorporated in the orthodontic bonding resin (Transbond XT) Test results were recorded according to the following groups:
Group 1: Control group: Transbond XT
Group 2: Experimental group: Transbond XT with 5 wt% AgO nanoparticles
Group 3: Experimental group: Transbond XT with 10 wt% AgO nanoparticles.
These groups were subjected to both Antibacterial and shear bond strength evaluation.
The results and the static values are enumerated in the form of tables and graph.
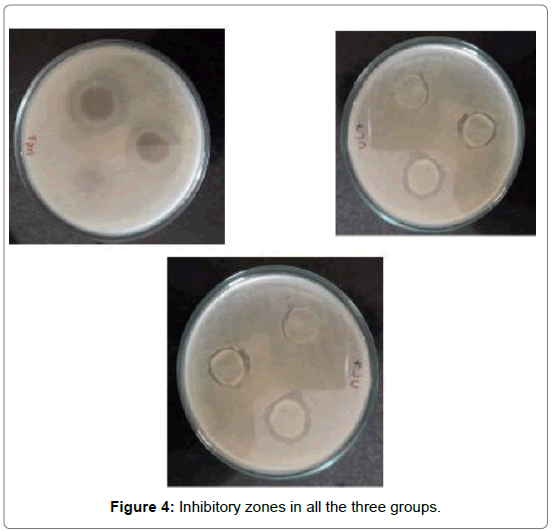
The ability of the modified composite to inhibit growth of Streptococcus mutans are listed in Table 1. The inhibitory activity was measured based on the diameter of the clear inhibition zone. Table 1 denotes the mean and standard deviation of the inhibitory zone among all the groups In group I i.e Transbond XT the mean inhibitory zone was found to be 1.63 mm with a standard deviation of 0.23. For group II i.e. (Transbond XT with 5 wt% AgO nanoparticles) the mean inhibitory zone was found to be 1.84 mm with a standard deviation of 0.30.
| Diameter(mm) of inhibitory zone against S.Mutans | |||||
|---|---|---|---|---|---|
| Group | N | Mean | Std. Deviation | Minimum | Maximum |
| Control_Group_TransbondXT | 10 | 1.63 | 0.23594 | 1.2 | 1.9 |
| Composite Transbond XT with 5percent Ag | 10 | 1.84 | 0.30984 | 1.2 | 2.1 |
| Composite Transbond XT with 10percentAg | 10 | 2.79 | 0.27669 | 2.4 | 3.2 |
| Total | 30 | 2.0867 | 0.57819 | 1.2 | 3.2 |
Table 1: Antibacterial activity.
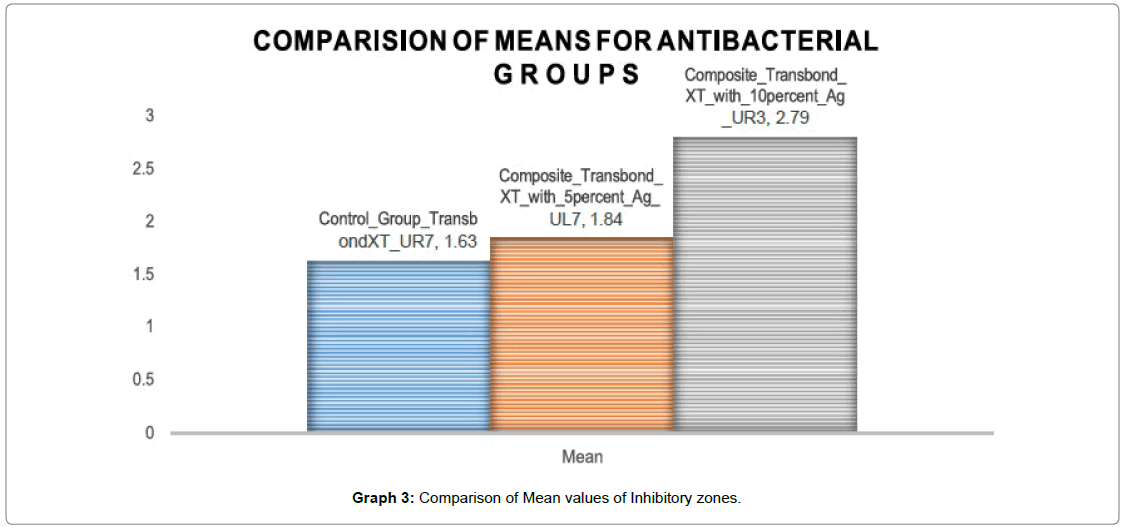
For group III i.e. (Transbond XT with 10 wt% AgO nanoparticles) the mean inhibitory zone was found to be 2.79 mm with a standard deviation of 0.27. The mean was found to be highest for Group III i.e. (Transbond XT with 10 wt% AgO nanoparticles) (2.79 mm) followed by Group II i.e. (Transbond XT with 5 wt% AgO nanoparticles) (1.84 mm) and least for group III (Transbond XT) (1.63 mm).
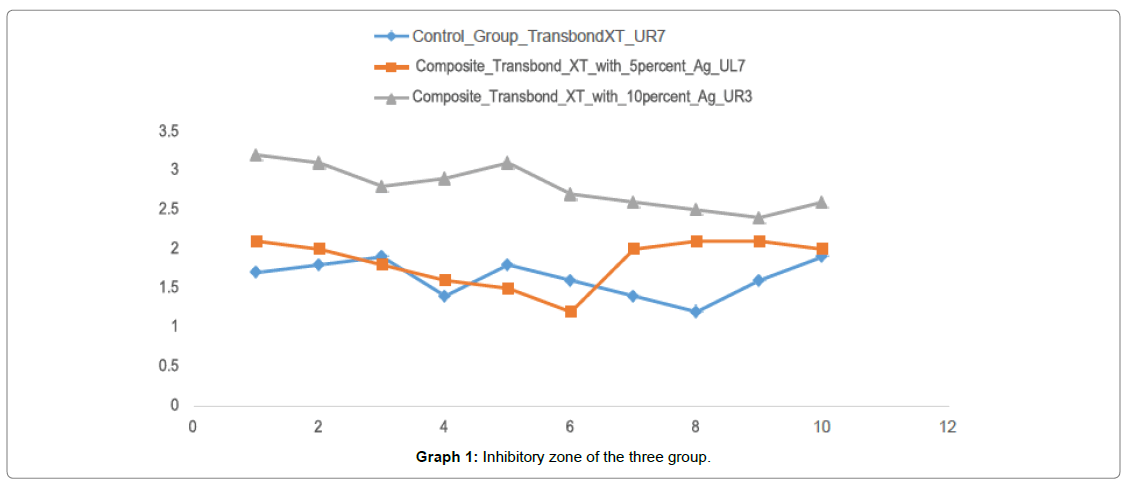
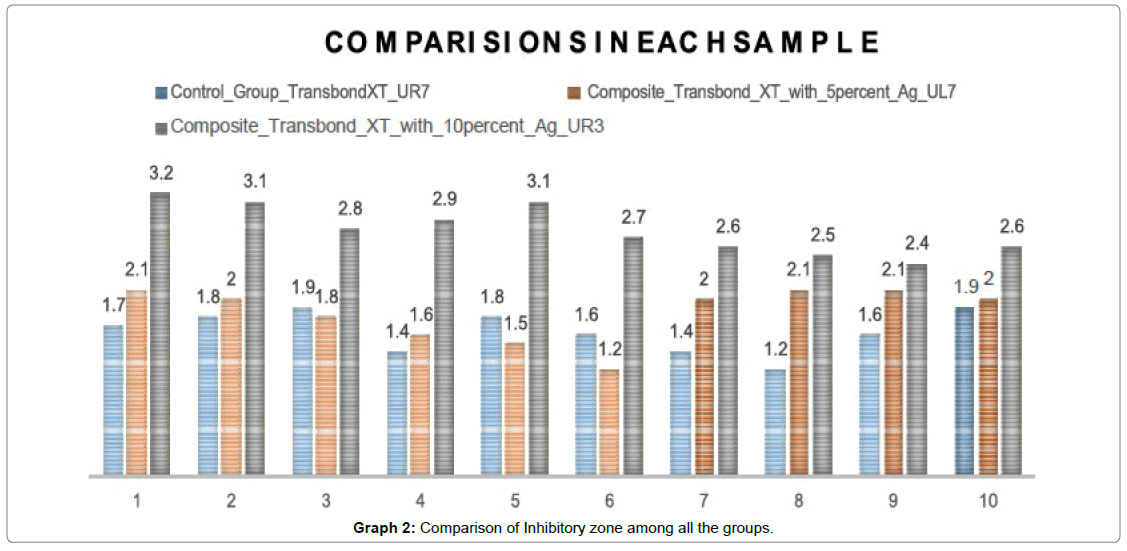
Graph 1 shows the inhibitory zone of all the three groups and Group III (Transbond XT with 10 wt% AgO nanoparticles) has shown the highest inhibitory zone as compared to the control group with the least inhibitory zone.
Table 2 denotes the results of the comparison of the means of inhibitory zone among all the groups using one way ANOVA test. There was a statistically significant difference between the groups with p value<0.0001. Thus it can be inferred that group III (Transbond XT with 10% AgO nanoparticles) has statistically significant higher inhibitory zone followed by group II and group I. The mean inhibitory zone was highest for group III when compared to the control group.
| N | Mean | Std. Deviation | On way ANOVA | |
|---|---|---|---|---|
| Transbond XT | 10 | 1.63 | 0.23 | p value |
| Transbond XT with 5% AgO | 10 | 1.84 | 0.3 | <0.0001 (Significant) |
| Transbond XT with 10% AgO | 10 | 2.79 | 0.27 |
Table 2: Comparison of the mean among all the groups using one way ANOVA test.
According to the Graph 2 results there were significant differences between the control group and experimental group in relation to the effect on Streptococcus mutans. The composite resins containing 5% silver nanoparticles and those containing 10% of silver nanoparticles exhibited a significant antibacterial activity against Streptococcus mutans [9-12].
The Antibacterial effect of incorporating 10% silver oxide nanoparticles into composite resin was significantly higher than that of incorporating 5% silver nanoparticles when compared to the control group.
Graph 3 shows the mean inhibitory zone of all the three groups and Group III (Transbond XT with 10 wt% AgO nanoparticles) has shown the highest inhibitory zone i.e (2.79 mm) as compared to the control group with the least inhibitory zone i.e (1.63 mm). Group II (Transbond XT with 5 wt% AgO nanoparticles) shows a mean inhibitory zone of 1.84 mm which is comparable to the control group.
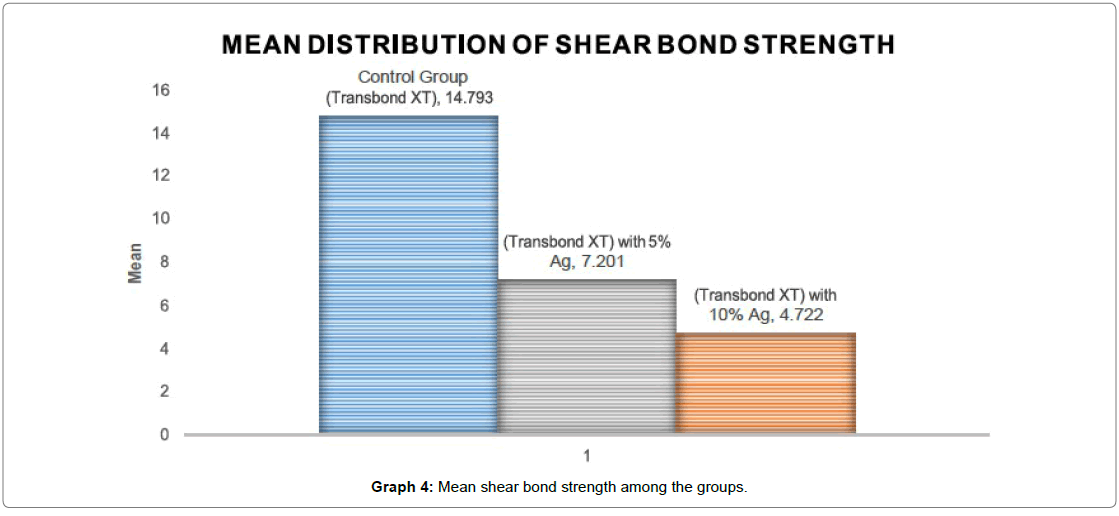
Table 3 denotes mean and standard deviation of the shear bond strength among all the groups. In Group I Control group : (Transbond XT), the mean bond strength is found to be 14.79 Mpa with a standard deviation of 3.04. For Group II (Transbond XT with 5% Ago nanoparticles), the shear bond strength is found to be 7.20 Mpa with a standard deviation of 0.92. Group III (Transbond XT with 10% Ago nanoparticles) had a mean shear bond strength of 4.72 Mpa with a standard deviation of 0.77. The mean was found to be the highest for Group I Control group Transbond XT (14.79 Mpa) followed by group II (Transbond XT with 5% Ago nanoparticles) (7.20 Mpa), and least for Group III (Transbond XT with 10% Ago nanoparticles) (4.72 Mpa).
| Shear Bond Strength | |||||
|---|---|---|---|---|---|
| Group | Mean | Std. Deviation | N | Minimum | Maximum |
| Control Group (Transbond XT) | 14.793 | 3.04647 | 10 | 10.34 | 20.46 |
| (Transbond XT) with 10% Ag | 4.722 | 0.77933 | 10 | 3.79 | 6.51 |
| (Transbond XT) with 5% Ag | 7.201 | 0.92278 | 10 | 6.22 | 9.23 |
| Total | 8.9053 | 4.72468 | 30 | 3.79 | 20.46 |
Table 3: Mean and standard deviation of Shear Bond Strength among groups.
Graph 4 denotes the mean shear bond strength of all the 3 groups, the mean SBS is found to be the highest for Group I Control group Transbond XT (14.79 Mpa) when compared to the other two groups.
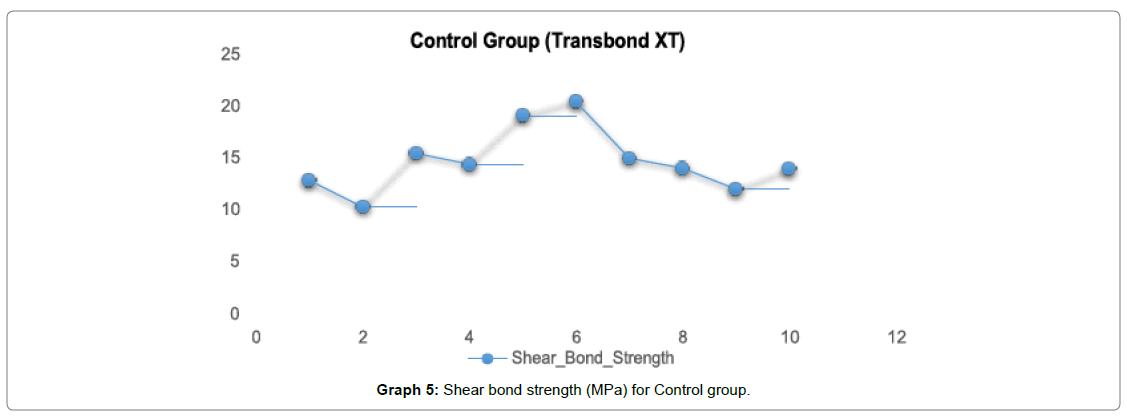
In Graph 5, for control group Transbond XT the shear bond strength ranged from 12-15 Mpa with a mean value of (14.79 Mpa).
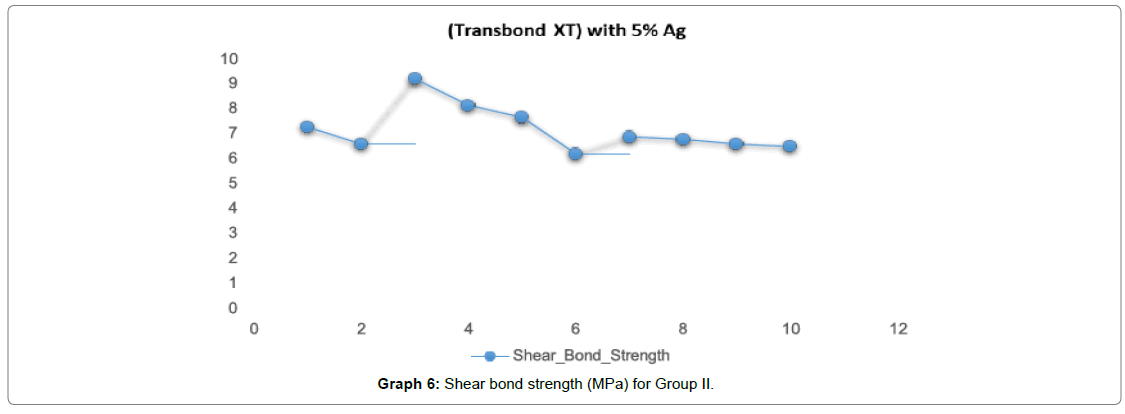
In Graph 6, for Group II (Transbond XT with 5% Ago nanoparticles) the shear bond strength ranged from 6-8 Mpa with a mean value of (7.20 Mpa).
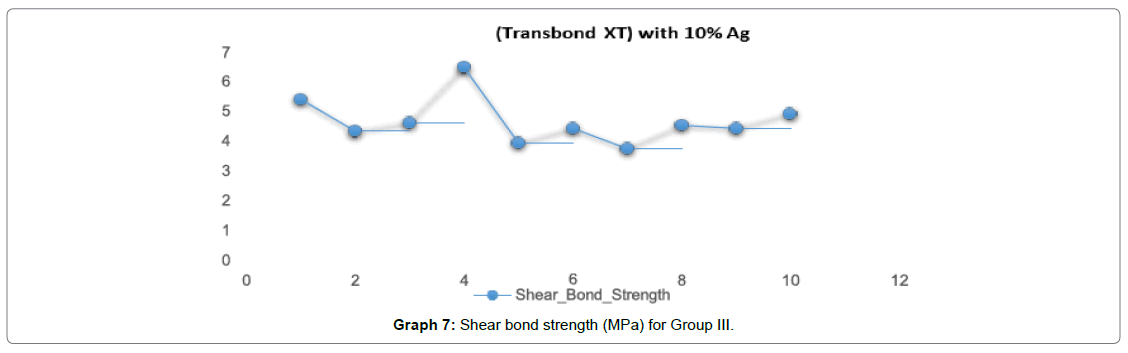
In Graph 7, for Group III (Transbond XT with 10% Ago nanoparticles) the shear bond strength ranged from 4-5 Mpa with a mean value of (4.72 Mpa).
10 samples in each group, for a total of three groups were tested for shear bond strength. The mean shear bond strength, standard deviation, minimum and maximum and a range of groups are shown in Table 3. A one way ANOVA test showed control group transbond XT were significantly higher in bond strength than the other two groups.
In Table 4, it indicates that the strength of association between the three groups is very high and that the correlation coefficient is very highly significant. In conclusion the Pearson correlation showed that all the three groups of antibacterial activity are inversely proportional to all the three groups of shear bond strength with a coefficient of 0.5 and they are significant at the 0.01 level which mean as the Percentage of Silver oxide Nanoparticles in the composite resin is increasing the antimicrobial activity in turn is increasing whereas the shear bond strength is decreasing. Therefore it can be concluded that adding 10% of Silver nano oxide particles to Transbond XT increases the Antibacterial activity but on the other hand it significantly reduces the Shear bond strength of Transbond XT with 10% Silver nano oxide particles.
| Correlations | |||||
|---|---|---|---|---|---|
| Column 1 | Column 2 | Antibacterial activity | Control Group (Transbond XT) | (Transbond XT) with 10% Ag | (Transbond XT) with 5% Ag |
| Shear Bond Strength | Pearson Correlation | 1 | .896** | -.637** | -0.259 |
| Sig. (2-tailed) | 0 | 0 | 0.166 | ||
| N | 30 | 30 | 30 | 30 | |
| Control Group (Transbond XT) | Pearson Correlation | .896** | 1 | -.500** | -.500** |
| Sig. (2-tailed) | 0 | 0.005 | 0.005 | ||
| N | 30 | 30 | 30 | 30 | |
| (Transbond XT) with 10% Ag | Pearson Correlation | -.637** | -.500** | 1 | -.500** |
| Sig. (2-tailed) | 0 | 0.005 | 0.005 | ||
| N | 30 | 30 | 30 | 30 | |
| (Transbond XT) with 5% Ag | Pearson Correlation | -0.259 | -.500** | -.500** | 1 |
| Sig. (2-tailed) | 0.166 | 0.005 | 0.005 | ||
| N | 30 | 30 | 30 | 30 | |
| **. Correlation is significant at the 0.01 level (2-tailed). | |||||
Table 4: Pearson correlation of Antibacterial activity and Shear Bond Strength among all the groups.
Discussion
Bacterial biofilms are responsible for dental diseases, such as caries and periodontitis. Orthodontic bonding materials may be regarded as artificial predilection site for the accumulation of oral micro-organisms which leads to formation of incipient caries, commonly called white spot lesions (WSLs), which is an unaesthetic, common side effect of orthodontic treatment. White spot lesions (WSLs) is caused by increased numbers of Streptococcus mutans and other pathological microbes in the biofilm, decreased pH and compromised oral hygiene. Consequently the control of bacterial adhesion and biofilm formation on dental materials is now understood as a crucial factor; therefore the development of composites that exhibit bactericidal activities is necessary. Application of nanoparticles of silver is increasingly growing in Orthodontic material and has shown significant effects in terms of antimicrobial properties. An appropriate concentration of antimicrobial agents is important because insufficient amounts limit antimicrobial activity and excess amounts deteriorate the mechanical properties of orthodontic materials. Due to the potential of composite resin to attract micro-organisms the present study made an attempt to evaluate the antibacterial properties and shear bond strength of composite resin containing silver nano oxide particles [13-23]. The findings of this study indicates that the Antibacterial activity of Transbond XT with 10% of silver oxide nanoparticles is significantly higher when compared to those of Transbond XT with 5% of silver oxide nanoparticles and the control group with Transbond XT. When a comparison was done among the groups, a statistically significant difference is found fo the Antibacterial activity (Inhibition zone). Among the three groups the Antibacterial activity against Streptococcus mutans was found to be highest for Group III Transbond XT with 10 wt% AgO nanoparticles (2.79 mm) followed by Group II Transbond XT with 5 wt% AgO nanoparticles (1.84 mm) and least for group III Transbond XT. Because of their small size, AgNPs possess chemical physical and biological properties distinctive from those presented by traditional bulk materials. Their smaller particles and large surface area provide potent antibacterial effects at a low filler level. Other advantage provided by the small size is the possibility of AgNPs to penetrate through cell membranes more readily, resulting in higher antimicrobial activity. Most of the previous studies confirm the antibacterial properties of Nano silver incorporated into composite resins; however they have reported different concentrations and effectiveness of these particles [18-20]. Yoshida et al. demonstrated the antibacterial activity of composite resin containing high concentrations of silver nanoparticles (up to 10%) against Streptococcus mutans [20]. Hernandez – sierra et al. evaluated the sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide and gold and indicated the higher antibacterial properties of Nano silver than Nano zinc oxide and Nano gold. Mohadese Azarsina et al. evaluated the antibacterial properties of composite resin containing nanosilver with 0.5 and 1% by weight which significantly inhibited the growth of Streptococcus mutans and lactobacillus.
Our results show that AgO-NPs cause inhibition of bacterial biofilm which is more prominent with higher concentrations [24-39]. The 10% group significantly reduced S. mutans growth as compared to the 5% group and the control group. The diameter of inhibition was highest for 10% group among all the three groups. It was found that higher the concentration of Silver Nano oxide particles greater the antibacterial activity was against Streptococcus mutans. The results for the shear bond strength showed that incorporation of silver oxide nanoparticles decreases the shear bond strength of composite resin. Incorporation of silver oxide nanoparticles containing 5% of silver reduces the bond strength to a level comparable to the control group. Also finally further increasing the amount of silver nanoparticles to the 10% level demonstrates a decline in the shear bond strength. The mean shear bond strength was found to be the highest for Group I Control group Transbond XT (14.79 Mpa) followed by group II (Transbond XT) with 5% Ag (7.20 Mpa), and least for Group III (Transbond XT) with 10% Ag (4.72 Mpa). Azam Akhavan et al. investigated the effect of incorporating 1%, 5% and 10% nanosilver into composite resin and found that nanoparticles containing 5% and 1% silver maintains and increases the SBS of orthodontic adhesives respectively whereas 10% of silver decreases the Shear bond strength [11,40-44].
The decrease in bond strength associated with the addition of more nanoparticles could be due to an agglomeration of particles, creating defect points and interfering with the curing process of the hydroxyapatite nanoparticles, which in theory can be proposed to enhance remineralisation of the enamel surface located around the bracket and consequently aid in the prevention of dental caries [11].The composites used in this study were not mixed with the nanoparticles by a mechanical mixer, due to their high viscosity. The manual incorporation of the nanoparticles into the composite resin may include air bubbles into the composite, which might affect some of its properties. Although the composites were completely packed in the moulds during sample preparation, and despite the observation that these porosities did not have any deleterious effect on the antibacterial properties of the studied composites, it might have affected the mechanical properties. Many investigations dealing with the orthodontic shear bond strength of adhesives compare the results of their studies with previously published articles, claiming that a value between 6-8 Mpa is a clinically acceptable figure, adequate to withstand occasional masticatory loads and at the same time low enough to prevent damage to the enamel [11]. Therefore in order to avoid misjudgements, it appears more logical to discuss the results of this study within its own boundaries. Based on this concept and giving consideration to the opinions of some who propose Transbond XT as the=gold standard‘, it could be proposed that incorporation of 5% of silver oxide nanoparticles keeps the bond strength at the same=clinically ‘acceptable level (6-8 Mpa) Further increasing it to 10% decreases the shear bond strength [11,45-50]. Incorporating nanoparticles into the bonding agent and not into the adhesive body itself could be considered more logical approach and somehow beneficial from a clinical viewpoint, since it is the bonding agent which comes into direct contact with the enamel surface: the target area for preventive attempts. Further studies are undoubtedly required to investigate the biological effects of adding such amounts of nanoparticles.
Conclusion
The present study was carried out to evaluate the anti-bacterial property and shear bond strength of silver oxide nanoparticles incorporated in the orthodontic bonding agents. The results of the study revealed the following:
Findings
Addition of Silver oxide nanoparticles to Transbond XT could significantly inhibit the growth of Streptococcus mutans. When compared to the control group, the group with 10% silver nano oxide particles exhibited higher antibacterial activity than the control group and the group containing 5% silver nano oxide particles. It was observed that the addition of silver oxide nanoparticles to Transbond XT affects the shear bond strength of the product. The incorporation of 5% silver oxide nanoparticles reduces the shear bond strength but is within the clinically acceptable figure (6-8 Mpa). The incorporation of 10% silver oxide nanoparticles has an undesirable effect on shear bond strength when compared to the control group.
The limitations of the study were that the composites used in this study were not mixed with the nanoparticles by a mechanical mixer, due to their high viscosity. The manual incorporation of the nanoparticles into the composite resin may include air bubbles into the composite, which might affect some of its properties. Discoloration and greyish colour change is a common problem in all materials containing silver, especially composite resins. Silver nanoparticles create a dark grey colour change in composite and are not suitable for orthodontic bonding applications and also are unaesthetic for bonding esthetic orthodontic appliance such as ceramic brackets.
Suggestions for further research
AgNPs containing composites present good antimicrobial properties, however much is still to be discovered. One of the most important experiments to be performed is to apply the bench results on in vivo studies, since laboratory conditions do not exactly reproduce oral conditions. Addition of Silver oxide nanoparticles in small proportions is very beneficiary for both antibacterial activity as well as shear bond strength. Silver oxide nanoparticles with different proportions like 1%, 2%, 3% and 4% can be added to the composite resin for improved bactericidal results. Other aspect to be investigated is the long term effectiveness of AgNPs in composites, whereas a long lasting antimicrobial potential of them is desirable.
References
- Jedrychowski JR, Caputo AA, Kerper S (1983) Antibacterial and mechanical properties of restorative materials combined with chlorhexidines. J Oral Rehabil 10: 373-381.
- Underwood ML, Rawls HR, Zimmerman BF (1989) Clinical evaluation of a fluoride exchanging resin as orthodontic adhesive. Am J Orthod Dentofacial Orthop 96: 93-99.
- Sonis AL, Snell W (1989) An evaluation of a fluoride-releasing, visible light-activated bonding system for orthodontic bracket placement. AM J Orthod Dentofac Orthop 306-311.
- Chan DC, Swift EJ Jr, Bishara SE (1990) In vitro Evaluation of a Fluoride-releasing Orthodontic Resin. J Dent Res 69: 1576-1579.
- Wilson SJ, Wilson HJ (1993) The release of chlorhexidine from modified dental acrylic resin. J Oral Rehabil 20: 311-319.
- Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RR (1994) Incorporation of Bacterial Inhibitor into Resin Composite. J Dent Res 73: 1437-1443.
- Imazato S, Russell RR, McCabe JF (1995) Antibacterial activity of MDPB polymer incorporated in dental resin. J Dent 23: 177-181.
- Wiltshire WA, Janse van Rensburg SD (1995) Fluoride release from four visible light-cured orthodontic adhesive resins. Am J Orthod Dentofacial Orthop 108: 278-283.
- Ashcraft DB, Staley RN, Jakobsen JR (1997) Fluoride release and shear bond strengths of three light-cured glass ionomer cements. Am J Orthod Dentofac Orthop 111: 260-265.
- Imazato S, Ehara A, Torii M, Ebisu S (1998) Antibacterial activity of dentine primer containing MDPB after curing. J Dent 26: 267-271.
- Akhavan A, Sodagar A, Mojtahedzadeh F, Sodagar K (2013) Investigating the effect of incorporating nanosilver/nanohydroxyapatite particles on the shear bond strength of orthodontic adhesives. Acta Odontologica Scandinavica 71: 1038-1042.
- Kassaee MZ, Akhavan A, Sheikh N, Sodagar A (1998) Antibacterial Effects of a New Dental Acrylic Resin Containing Silver Nanoparticles. J Applied Polym Sci 110: 1699-1703.
- Manfred L, Covell DA, Crowe JJ, Tufekci E, Mitchell JC (1998) A novel biomimetic orthodontic bonding agent helps prevent white spot lesions adjacent to brackets. Angle Orthod 83: 97-103.
- Ewoldsen N, Demke RS (1998) A review of orthodontic cements and adhesives. Am J Orthod Dentofacial Orthop 120: 45-48.
- Ahn SJ, Lee SJ, Kook JK, Lim BS (1998) Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles Dental materials 25: 206-213.
- Mohamed hamouda (1998) Current persepectives of nanoparticles in medical and dental biomaterials. J Biomed Res 26: 143-151.
- Correa JM, Mori M, Sanches HL, da Cruz AD, Poiate E Jr, et al. (2015) Silver nanoparticles in dental biomaterials. Int J Biomater 2015: 485275.
- Tanagawa M, Yoshida K, Matsumoto S, Yamada T, Atsuta M (1999) Inhibitory Effect of Antibacterial Resin Composite against Streptococcus mutans. Caries Res 33: 366-371.
- Hotta M, Nakajima H, Yamamoto K, Aono M (1998) Antibacterial temporary filling materials: the effect of adding various ratios of Ag-Zn-Zeolite. J Oral Rehabil 25: 485-489.
- Yoshida K, Tanagawa M, Atsuta M (1999) Characterization and inhibitory effect of antibacterial dental resin composites incorporating silver-supported materials. J Biomed Mater Res 47: 518-522.
- Othman HF, Wu CD, Evans CA, Drummond JL, Matasa CG (2002) Evaluation of antimicrobial properties of orthodontic composite resins combined with benzalkonium chloride. Am J Orthod Dentofacial Orthop 122: 288-294.
- Kumar R, Münstedt H (2005) Silver ion release from antimicrobial polyamide/silver composites. Biomaterials 26: 2081-2088.
- Eminkahyagil N, Korkmaz Y, Gokalp S, Baseren M (2005) Shear bond strength of orthodontic brackets with newly developed antibacterial self- etch adhesive. Angle Orthod 75: 843-847.
- Sehgal V, Shetty VS, Mogra S, Bhat G, Eipe M, et al. (2007) Evaluation of antimicrobial and physical properties of orthodontic composite resin modified by addition of antimicrobial agents--an in-vitro study. Am J Orthod Dentofacial Orthop 131: 525-529.
- Damm C, Munstedt H, Rosch A (2007) Long-term antimicrobial polyamide 6/silver- nanocomposites. J Mater Sci 42: 6067-6073.
- Casemiro LA, Gomes Martins CH, Pires-de-Souza Fde C, Panzeri H (2008) Antimicrobial and mechanical properties of acrylic resins with incorporated silver–zinc zeolite – part I. Gerodontology 25: 187-194.
- Hernández-Sierra JF, Ruiz F, Pena DC, Martínez-Gutiérrez F, Martínez AE, et al. (2008) The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine 4: 237-240.
- Kong H, Jang J (2008) Antibacterial Properties of Novel Poly(methyl methacrylate) Nanofiber Containing Silver Nanoparticles. Langmuir 24: 2051-2056.
- Pinto RJ, Marques PA, Neto CP, Trindade T, Daina S, et al. (2009) Antibacterial activity of nanocomposites of silver and bacterial or vegetable cellulosic fibers. Acta Biomaterialia 5: 2279-2289.
- Bürgers R, Eidt A, Frankenberger R, Rosentritt M, Schweikl H, et al. (2009) The anti-adherence activity and bactericidal effect of microparticulate silver additives in composite resin materials. Arch Oral Biol 54: 595- 601.
- Li LH, Deng JC, Deng HR, Liu ZL, Li XL (2010) Preparation, characterization and antimicrobial activities of chitosan/Ag/ZnO blend films. Chem Eng J 160: 378-382.
- Espinosa-Cristóbal LF, Martínez-Castañón GA, Martínez-Martínez RE, Loyola-Rodriguez JP, Patino-Marin N, et al. (2009) Antibacterial effect of silver nanoparticles against Streptococcus mutans. Materials Letters. 63: 2603-2606.
- Sadat-Shojai M, Atai M, Nodehi A, Khanlar LN (2010) Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: Synthesis and application. Dent Mater 26: 471-482.
- Kuroki K, Hayashi T, Sato K, Asai T, Okano M, et al. (2010) Effect of self-cured acrylic resin added with an inorganic antibacterial agent on Streptococcus mutans. Dent Mater J 29: 277-285.
- Cheng L, Weir MD, Xu HH, Antonucci JM, Kraigsley AM, et al. (2012) Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent Mater 28: 561-572.
- Melo MA, Cheng L, Zhang K, Weir MD, Rodrigues LK, et al. (2013) Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent Mater 29: 199-210.
- Cheng L, Zhang K, Melo MA, Weir MD, Zhou X, et al. (2012) Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. J Dent Res 91: 598-604.
- Poosti M, Ramazanzadeh B, Zebarjad M, Javadzadeh P, Naderinasab M, et al. (2013) Shear bond strength and antibacterial effects of orthodontic composite containing TiO2 nanoparticles. Eur J Orthod 35: 676-679.
- Azarsina M, Kasraei S, Yousef-Mashouf R, Dehghani N, Shirinzad M (2013) The antibacterial activity of composite resin containing nanosilver against streptococcus mutans and lactobacillus. J Contempt Dent Pract 14: 1014-1018.
- Liu T, Song X, Guo Z, Dong Y, Guo N, et al. (2013) Prolonged antibacterial effect of silver nanocomposites with different structures. Colloids Surf B Biointerfaces 116: 793-796.
- Cheng L, Zhang K, Weir MD, Liu H, Zhou X, et al. (2013) Effects of antibacterial primers with quaternary ammonium and nano-silver on Streptococcus mutans impregnated in human dentin blocks. Dent Mater 29: 462-472.
- Zhang K, Li F, Imazato S, Cheng L, Liu H, et al. (2013) Dual antibacterial agents of nano-silver and 12- methacryloyloxydodecylpyridinium bromide in dental adhesive to inhibit caries. J Biomed Mater Res B Appl Biomater 101: 929-938.
- Tavassoli Hojati S1, Alaghemand H, Hamze F, Ahmadian Babaki F, Rajab-Nia R, et al. (2013) Antibacterial, physical and mechanical properties of flowable resin composites containing zinc oxide nanoparticles. Dent Mater 29: 495-505.
- Song J, Kim H, Jang Y, Jang J (2013) Enhanced antibacterial activity of silver/polyrhodanine-composite- decorated silica nanoparticles. ACS Appl Mater Interfaces 5: 11563-11568.
- Kasraei S, Sami L, Hendi S, AliKhani MY, Rezaei-Soufi L, et al. (2014) Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor Dent Endod 39: 109-114.
- Al-Husayni OK, Hatoor NA (2014) Effect of silver nitrate incorporation into heat polymerized acrylic resin on some mechanical properties. J Bagh Coll Dent 26: 78-85.
- Han Z, Zhu B, Chen R, Huang Z, Zhu C, et al. (2015) Effect of silver-supported materials on the mechanical and antibacterial properties of reinforced acrylic resin composites. Mater Design 65: 1245-1252.
- Pérez-Díaz MA, Boegli L, James G, Velasquillo C, Sánchez-Sánchez R, et al. (2015) Silver Nanoparticles with antimicrobial activities against streptococcus mutans and their cytotoxic effect. Mater Sci Eng C Mater Biol Appl 55: 360-366.
- Chung SH, Cho S, Kim K, Lim BS, Ahn SJ (2017) Antimicrobial and physical characteristics of orthodontic primers containing antimicrobial agents. Angle Orthod 87: 307-312.
- Wu J, Weir MD, Melo MA, Xu HH (2015) Development of novel self-healing and antibacterial dental composite containing calcium phosphate nanoparticles. J Dent 43: 317-326.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi