Research Article, J Clin Nutr Metab Vol: 1 Issue: 2
Effect of Vitamin D Supplementation for Obese Pregnant Women on Gestational Diabetes and Diabetes Biomarkers
Faten Tamim1*, Hamed Takruri1, Kamil Aframb2, Feda’a Thikrallahb2, Maisa’a Al-Khadrab2 and Asma Al-Bashab2
1Department of Dietetics and Nutritional Science, Faculty of Agriculture, University of Jordan, Jordan
2Department of Obstetrics and Gynecology, Jordan University Hospital, Jordan
*Corresponding Author : Faten Tamim
Department of Nutrition and Food Processing, Faculty of Agriculture, University of Jordan, Jordan
Tel: +00962798839787
E-mail: fatintamim@yahoo.com
Received: November 06, 2017 Accepted: November 27, 2017 Published: December 03, 2017
Citation: Tamim F, Takruri H, Aframb K, Thikrallahb F, Al-Khadrab M, et al. (2017) Effect of Vitamin D Supplementation for Obese Pregnant Women on Gestational Diabetes and Diabetes Biomarkers J Clin Nutr Metab 1:2.
Abstract
Background: High rates of vitamin D deficiency in Jordan are alarming especially when they are associated with obesity during pregnancy.
Objectives: To investigate the effect of vitamin D supplementation for obese pregnant women on gestational diabetes and diabetes biomarkers.
Methods: 118 women were investigated and were divided into three groups. Each group was divided into two treatment subgroups. (1) Women (n=23) with normal 25(OH)D levels were given either no supplementation (1A=12) or given vitamin D supplementation of 10000 IU/wk (1B=11), (2) women (n=43) with insufficient 25(OH)D levels were given either 10000 IU/wk (2A= 22) or 20000 IU/wk of vitamin D supplementation (2B=21), (3) women of group 3 (n=52) with deficient 25(OH)D levels were given either 20000 IU/wk (3A=26) or 50000 IU/wk (3B=26) of vitamin D supplementation.
Results: Fasting blood sugar showed decreased levels among B treatment subgroups in both group 1 and 3, while no significant difference was found between A and B treatment subgroups in group 2. Insulin resistance showed a significant difference between A and B treatment subgroups among group 2 and 3 but not group 1.
Conclusion: Screening for 25(OH)D during pregnancy and appropriate replacement, especially in patients with severe deficiency, may contribute to the prevention of gestational diabetes mellitus.
Keywords: Vitamin D, Obesity, Gestational diabetes, Insulin
Introduction
Vitamin D is a fat soluble hormone that is derived from cholesterol and comes primarily from skin exposure to sunlight. The nutritional forms of vitamin D include vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol); however, both forms are biologically inactive. Vitamin D2 is a plant sterol that composes 20% of systemic vitamin D and can be obtained from a plant based diet, while vitamin D3 composes 80% of systemic vitamin D [1] and can be obtained from sun light or from few foods of animal origin [2]. More than 90% of vitamin D3 is synthesized from epidermal 7-dehydroxycholesterol when exposed to ultraviolet B (UVB) photons [3]. Several studies were performed to investigate the association between serum 25(OH) D levels with blood glucose levels, insulin sensitivity, and adiposity biomarkers among both lean and obese individuals. However, research limitations sometimes induce controversies in the results of these investigations. A cross sectional study investigated the relationship between serum 25(OH)D and adiposity, glucose level, lipid profile, and adiponectin in 381 healthy university students (201 males and 180 females) [4]. The investigation showed a significant inverse relationship between serum 25(OH)D levels and fasting blood glucose (FBG), insulin levels and homeostasis model assessment of insulin resistance (HOMA index) [4]. Contrary to previous study, it was found in a randomized clinical trial of 45 pre-diabetes patients, aged between 33 and 61 years, that vitamin D had no effect on insulin sensitivity in pre-diabetes patients in 12 week treatment period with vitamin D supplementation [5].
In a cross sectional study that investigated the relationship between low maternal serum 25(OH)D levels and gestational diabetes among Turkish pregnant women; it was found that women with gestational diabetes had a significantly lower 25(OH)D levels (P<0.0001) than controls [6]. The results of study indicated that gestational diabetes was more common only in women with severe deficiency of serum 25(OH)D levels even after adjusting for established risk factors for gestational diabetes [6]. These results may indicate that the investigation of serum 25(OH)D levels during pregnancy and appropriate replacement, especially in women with severely deficient levels, may contribute to gestational diabetes prevention and thus its corresponded adverse pregnancy outcomes. Another study examined the prospective associations of baseline serum 25(OH)D with glucose homeostasis and insulin resistance in 489 individuals at high risk for type 2 diabetes mellitus (T2DM) [7]. The study indicated a significant association between baseline serum 25(OH)D levels and 3 years follow up of oral glucose tolerance test (OGTT) and homeostasis model assessment of insulin resistance (HOMA-IR). Higher baseline serum 25(OH)D independently reduced the progression of dysglycemia after 3 years of follow up suggesting a potential role of vitamin D in the etiology of T2DM.
Many studies suggested that vitamin D supplementation for obese and prediabetes individuals may contribute to prevention of diabetes and insulin resistance. In a randomized double blind controlled trial, the researcher examined the effect of vitamin D supplementation compared with placebo on pancreatic β-cell function, insulin sensitivity and glycaemia in participants at risk for T2DM [8]. One hundred thirty vitamin D deficient participants were grouped into a group taking vitamin D 400 IU three tablets per day or a group on identical placebo tablets per day and followed up for 16 weeks. After 4 months of treatment, serum 25(OH)D significantly increased in the vitamin D supplementation group compared with placebo group (PË‚0.001). However; no significant difference was found between the 2 groups in insulin sensitivity and β-cell function. Improved insulinogenic index was found in participants with serum 25(OH)D level ≥ 60 nmol/L, suggesting better effect with higher doses of vitamin D supplementation.
Materials and Methods
Study design
The study was designed as a randomized controlled double-blind trial that examined the effect of vitamin D supplementation for obese pregnant women on gestational diabetes and diabetes biomarkers. The trial was conducted at the Obstetrics and Gynecology Clinics at The Jordan University Hospital from March 2016 to October 2016. It was conducted according to the guidelines of the Declaration of Helsinki and all procedures involving human subjects were approved by the Committee of Ethics in Medical Research. A written informed consent was obtained from all participants.
Sampling
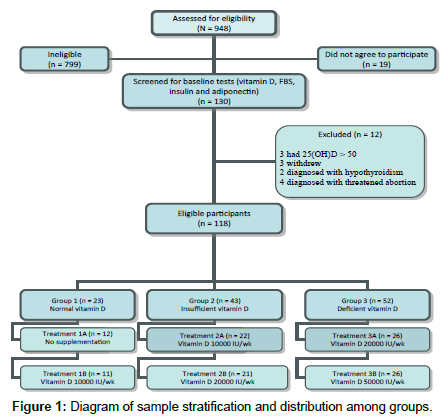
Each participant was interviewed at enrollment to fulfill a wellstructured 2-page questionnaire for socio-demographic data and medical history. The questionnaire contained questions including age, education, parity, occupation, annual income, pre pregnancy and current weight and height, previous and current medical, surgical and obstetrical history, smoking, medication use and supplements intake. Ninety hundred and forty eight pregnant women were assessed for eligibility of which 799 were found ineligible and 19 did not agree to participate. The remaining 130 participant were randomly recruited in the trial using computer generated random numbers of 3 digits in 948 blocks.
Inclusion and exclusion criteria
Inclusion criteria included singleton pregnancy, with 16-18 weeks gestational age, obese with BMI ≥ 30 kg/m2, and age range from 20 to 35 years old. Exclusion criteria included taking vitamin D supplementation more than 400 IU/d prior to enrollment, high risk pregnancies (preterm delivery during an earlier pregnancy, intrauterine growth retardation, pregnancy-induced hypertension, gestational diabetes, smokers, those with history of stillbirth or fetal death, mothers of twins pregnancy, those with history of polycystic ovary syndrome, and mothers of in vitro fertilization (IVF) pregnancy), known with major fetal anomaly, a concurrent interfering medications (i.e., corticosteroids, diuretics or cardiac medications including calcium channel blockers), chronic medical conditions such as diabetes mellitus, hypertension, renal disease, liver disease, parathyroid or active thyroid disease (e.g., Graves, Hashimoto’s or thyroiditis), congenital heart disease and serum level of 25(OH)D ≥ 50 ng/ml.
Random assignment and blinding
Potential participant underwent screening for obesity by weight and height measurements and BMI calculations. The blood specimens for baseline measurement of 25(OH) D, FBS and insulin level were obtained at the enrollment time. Eligible participants were randomly assigned within 4-6 weeks after baseline screening (n=130) and were assigned to one of 3 main groups according to baseline serum 25(OH) D level. These groups were: group (1) who had normal serum 25 (OH) D levels ( ≥ 30-50 ng/ml), group (2) who had insufficient serum 25 (OH) D levels (21–29 ng/ml) and group (3) who had deficient 25 (OH) D levels ( ≤ 20 ng/ml). Envelopes of papers containing A or B types of treatment each were previously prepared for random assignment of women to either treatment A or treatment B within each main group. Twelve women were excluded due to multiple reasons which were: 3 had 25(OH) D˃50, 3 women withdrew, 2 women were diagnosed with hypothyroidism and 4 women were diagnosed with threatened abortion. The eligible women (n=118) were stratified into subgroups as follows (Figure 1); Group (1) were women who had normal serum 25(OH)D levels (n=23) and randomly assigned to either without vitamin D supplementation ([1A] n=12) or 10000 IU/wk vitamin D supplements ([1B]n=11) . Group (2) were women who had insufficient serum 25(OH)D levels (n=43) and received either vitamin D 10000 IU/wk ([2A] n=21) or vitamin D 20000 IU/ wk ([2B] n=22). Group (3) were women who had deficient serum 25(OH)D levels (n=52) and received either vitamin D 20000 IU/wk ([3A]n=26) or vitamin D 50000 IU/wk ([3B] n=26). Supplement capsules were removed from the original container and kept in a healthy pack without revealing capsule dose for both provider and participant.
Each woman was followed up at each antenatal visit from enrollment up to delivery which was about 5 months. The participant compliance with intervention scheme was evaluated by maternal selfreport and pill count at each follow up visit. Women were reminded of supplement using text messages or phone calls.
Vitamin D supplements
Each participant was given vitamin D3 in a form of soft gelatin capsules containing either 10000 IU equivalent to 0.25 mg or 50000 IU equivalent to 1.25 mg vitamin D3 (cholecalciferol) in a digestible oil base. The capsule ingredients are vitamin D3, non-genetically modified (GMO) soybean lecithin, and bovine gelatin. The capsules are manufactured, packaged and warehoused in JFDA registered facilities. The supplement was registered under the name of Hi-Dee and produced by United Pharmaceuticals Manufacturing Co. Ltd, Amman – Jordan.
Capsules were given to each participant weekly on a regular basis to avoid effect of bias. Capsules were either one 10000 IU per week or 2 capsules of 10000 IU each per week or one capsule of 50000 IU per week according to treatment group.
Blood collection and analysis
Blood samples were collected on the day of enrollment as a base line assessment for the participants. The study duration was about 22– 24 week for each participant. At each follow up visit, participants were asked about any illness, medication use and tolerance of vitamin D supplement or drop of any of vitamin D doses. Venous blood samples (5 ml) were collected into serum-separating tubes (SST) which contain a special gel that separates the blood cells from serum when centrifuged. Blood samples were immediately centrifuged at 5000 g at 4°C for 10 min and kept in ice to be sent to Mega Laboratories (Amman) for analysis.
Determination of gestational diabetes
All pregnant women were screened for fasting blood glucose using the standard criteria by the American Diabetes Association (2016) considering fasting blood glucose (FBG) ≥ 126 mg/dl (7 mmol/L) as the cutoff point for the diagnosis of diabetes [9]. Screening for gestational diabetes was performed between the 28th and 34th weeks pregnancy using the oral glucose tolerance test (OGTT) after an overnight fasting (8-12 hours). Blood samples for fasting blood glucose were obtained, and then women were given a 75 gm glucose load followed by blood sampling after 1 and 2 hours for diagnosing blood glucose threshold. The diagnosis of gestational diabetes was considered when two or more blood glucose values met the criteria for a positive test: Fasting ≥ 95 mg/dL (5.3 mmol/L) and either 1-h postprandial ≥ 180 mg/dL (10 mmol/L) or 2-h post prandial ≥ 155 mg/dL (8 mmol/L) or both. Cut-off point for insulin level is 2.6-24.9 mcIU/ml and higher than 24.9 mc IU/ml is considered as insulin resistance. Homeostatic model assessment for insulin resistance (HOMA-IR) is an indicator of β-cell function and insulin resistance index [10]. It was estimated from serum fasting glucose and serum insulin levels using HOMA-IR calculator developed by Oxford Center for Diabetes, Endocrinology and Metabolism. Gestational diabetes was determined after being confirmed by the obstetrician on the medical record of the pregnant woman.
Statistical analysis
Data were analyzed using Statistical Package for Social Science (SPSS) program (version 20). The descriptive statistics were used to measure the percentage of women in each vitamin D group, mean age, mean pre pregnancy weight, height, weight at enrollment and BMI, mean gravidity and parity, mean BP, mean gestational age at enrollment and at delivery, mean gestational weight gain, and mean birth weight and height for each group separately. Continuous variables were presented as mean ± standard deviation (SD), P-value for differences between vitamin D groups and percentages used to represent the categorical variables. The Kolmogorov-Smirnov test was used to determine the normality of data. Differences in characteristics distribution between vitamin D groups were tested using Pearson x2 test for categorical variables and one-way ANOVA for continuous variables. The effect of maternal vitamin D supplementation on serum 25(OH)D levels, insulin level, HOMA-IR, blood sugar and infant 25(OH)D were analyzed using independent sample t -test. All data were tested to confirm normality of distribution and relationships between variables using simple linear regression models. A logistic regression analysis was applied to determine the association between vitamin D status and the occurrence of complications during pregnancy. Differences at P value ≤ 0.05 were considered statistically significant in all analyses.
Results
Socio demographic characteristics of study population
In Table 1, the characteristics of participants are given for each vitamin D category group. It can be noticed that there were no significant differences in age mean (in years) between category group 1 (29.39 ± 3.9), group 2 (29.77 ± 4.01) and group 3 (28.77 ± 4.38) (P˃0.30). Prepregnancy BMI was significantly higher in women of group 3 than those in group 1 and group 2. No significant difference in mean age was found in treatment subgroups (A and B) as well. Educational level showed no significant difference between the three main groups (P˃0.93). The three study groups have been more likely to include participants with university degree (61%, n=73). Employment showed no significant difference between the 3 groups and 44.5%, n=53 were employed and 55.5%, n=65 were unemployed. The majority of participants were non-smokers (95.8%, n=113). Most of participant had income below 2400 JDs/year (34.7%, n=41) with no significant difference between the 3 groups (P>0.82).
| Characteristics | All (n = 118 (%)) | Group 1 Sufficient 25(OH)D n = 23 (%) | Group 2 Insufficient 25(OH)D n = 43 (%) | Group 3 Deficient 25(OH)D n = 52 (%) | P – value |
|---|---|---|---|---|---|
| Age (y) (mean ± SD) | 29.31 ± 4.09 | 29.39 ± 3.9 | 29.77 ± 4.01 | 28.77 ± 4.38 | 0.30 |
| 20 – 25 | 21 (17.8%) | 0 (0%) | 9 (42.9%) | 12 (57.1%) | |
| 26 – 30 | 47 (39.8%) | 5 (10.6%) | 20 (42.6%) | 22 (46.8%) | |
| 31 – 35 | 50 (42.4%) | 7 (14%) | 25 (50%) | 18 (36%) | |
| Education | |||||
| Illiterate | 1 (0.8%) | 1 (100%) | 0 (0%) | 0 (0%) | 0.93 |
| Elementary | 5 (4.2%) | 0 (0%) | 3 (60%) | 2 (40%) | |
| Secondary | 34 (28.8%) | 2 (5.9%) | 19 (55.9%) | 13 (38.2%) | |
| University | 73 (61.9%) | 9 (12.3%) | 29 (39.7%) | 35 (47.9%) | |
| Higher education | 5 (4.2%) | 1 (20%) | 2 (40%) | 2 (40%) | |
| Work | |||||
| Employed | 53 (44.5%) | 6 (11.3%) | 21 (49.1%) | 26 (36.6%) | 0.67 |
| Not employed | 65 (55.5%) | 6 (9.2%) | 28 (43.1%) | 31 (47.7%) | |
| Income (JD/Year) | |||||
| < 2400 | 41 (34.7) | 5 (12.2%) | 19 (46.3%) | 17 (41.5%) | 0.82 |
| 2400 – 6000 | 45 (38.1) | 3 (6.7%) | 22 (48.9%) | 20 (44.4%) | |
| 6000 – 12000 | 7 (5.9%) | 0 (0%) | 3 (42.9%) | 4 (57.1%) | |
| Do not know | 25 (21.2%) | 4 (16%) | 10 (40%) | 11 (44%) | |
| Smoking | |||||
| Yes | 3 (2.5%) | 1 (33.3%) | 0 (0%) | 2 (66.7%) | 0.22 |
| No | 113 (95.8%) | 11 (9.7%) | 52 (46%) | 50 (44.2%) | |
| Occasionally | (2%) | 0 (0%) | 2 (100%) | 0 (0%) | |
Table 1: Frequency distribution of selected socio demographic characteristics of study population.
Anthropometrics, health indicators and baseline biomarkers: fasting blood sugar, insulin level, HOMA-IR and serum 25(OH)D level
Table 2 shows no significant association of the prepregnancy weight (kg) or height (cm) with vitamin D status, while BMI (kg/ m2) shows significant increase among participants of group 3 rather than group 1 or 2 (P ≤ 0.003). Baseline systolic and diastolic blood pressure (BP) showed no significant difference in means between groups. Gestational age ranged from 16 to 18 weeks of gestation with no significant difference in means between groups. Gravity showed no significant difference between groups while parity showed significantly higher parity among group 1 compared to group 2 and 3 (2.83 ± 1.89, 2.07 ± 1.5 and 2.04 ± 1.58 respectively) (P ≤ 0.05). A significant difference in the means of serum 25(OH)D levels is shown between group 1 (32.7 ± 9.4), 2 (24.1 ± 2.6) and 3 (14.1 ± 3.8) (P = 0.000). No significant difference was shown in FBS (mg/dl), insulin level (μU/ml) or HOMA-IR.
| Characteristics | Group 1 Sufficient 25(OH)D n = 23 (20%) (Mean ± SD) | Group 2 Insufficient 25(OH)D n = 43 (36%) (Mean ± SD) | Group 3 Deficient 25(OH)D n = 52 (44%) (Mean ± SD) | P - value |
|---|---|---|---|---|
| Gravida | 4.43 ± 2.31 | 3.47 ± 1.98 | 3.27 ± 1.84 | 0.06 |
| Parity | 2.83 ± 1.89* | 2.07 ± 1.5 | 2.04 ± 1.58 | 0.05 |
| Prepregnancy weight (Kg) | 78.4 ± 5.5 | 77.7 ± 7.8 | 78.7 ± 5.8 | 0.93 |
| Weight at enrollment (Kg) | 82.0 ± 5.8 | 81.9 ± 7.9 | 82.4 ± 1.4 | 0.94 |
| Height (cm) | 160.8 ± 6.4 | 169.6 ± 7.5 | 158.4 ± 7.5 | 0.23 |
| BMI (Kg/m2) | 30.43 ± 0.98 | 30.37 ± 0.96 | 31.26 ± 1.33* | 0.003 |
| Gestational Age at enrollment (wk) | 17.26 ± 1.4 | 16.79 ± 1.6 | 16.29 ± 1.4 | 0.41 |
| Systolic BP (mg/Hg) | 110 ± 6 | 109 ± 9 | 110 ± 11 | 0.09 |
| Diastolic BP (mg/Hg) | 63 ± 6 | 65 ± 8 | 65 ± 9 | 0.08 |
| 25(OH)D (ng/ml) | 32.7 ± 9.4* | 24.1 ± 2.6* | 14.1 ± 3.8* | 0.001 |
| Fasting blood sugar mg/dl | 84.93 ± 12.2 | 88.67 ± 14.2 | 88.55 ± 11.9 | 0.23 |
| Insulin level µU/ml | 15.0 ± 2.6 | 14.78 ± 2.4 | 14.76 ± 2.0 | 0.23 |
| HOMA – IR | 3.0 ± 9.5 | 3.3 ± 1.05 | 3.3 ± 0.9 | 0.30 |
Table 2: Baseline clinical and biological parameters of study participants.
Post-treatment pregnancy outcomes and biomarkers
Table 3 shows the results of pregnancy outcomes of both 1A and 1B treatment subgroups. Serum 25(OH)D was significantly increased in treatment subgroup 1B than treatment subgroup 1A (1A=29.0 ± 5.62 ng/ml, 1B = 39.30 ± 8.70 ng/ml, P ≤ 0.003). Fasting blood sugar was significantly decreased in treatment subgroup 1B (80.18 ± 8.95 mg/dl) compared to treatment subgroup 1A (102.90 ± 27.73 mg/dl) (P ≤ 0.01). Treatment subgroup 1B shows insignificant decreased mean HOMA-IR compared with treatment subgroup 1A (P ≤ 0.06). However, means of insulin level were significantly different (P ≤ 0.05). Cord 25(OH)D levels shows significant mean increase among those of treatment subgroup 1B (31.67 ± 7.06 ng/ml) compared to those of treatment subgroup 1A (21.80 ± 5.67 ng/ml) (P ≤ 0.001).
| Pregnancy outcomes | Treatment subgroup 1A n = 12 (Mean ± SD) | Treatment subgroup 1 B n = 11 (Mean ± SD) | P – value |
|---|---|---|---|
| 25(OH)D (ng/ml) | 29.0 ± 5.62 | 39.95 ± 8.90* | 0.003 |
| Fasting blood sugar mg/dl | 102.90 ± 27.73 | 80.18 ± 8.95* | 0.01 |
| Insulin level (µU/ml) | 17.17 ± 13.43 | 13.89 ± 11.94* | 0.05 |
| HOMA-IR | 4.89 ± 4.95 | 2.72 ± 2.22 | 0.06 |
| Cord 25(OH)D (ng/ml) | 21.98 ± 5.67 | 32.50 ± 7.06* | 0.001 |
Table 3: Pregnancy outcomes (diabetes biomarkers and cord 25(OH)D) in both 1A and 1B treatment subgroups.
Table 4 shows the results of pregnancy outcomes of both 2A and 2B treatment subgroups. Serum 25(OH)D was significantly increased in treatment subgroup 2B (34.67 ± 4.89 ng/ml) as compared with treatment subgroup 2A (25.81 ± 5.07 ng/ml) (P ≤ 0.001). Also, fasting blood sugar levels were decreased in treatment subgroup 2B (89.07 ± 12.96 mg/dl) in comparison with treatment subgroup 2A (107.55 ± 42.68 mg/dl) but the difference between them was statistically insignificant (P ≤ 0.06). In addition, a significantly decreased HOMAIR was found in treatment subgroup 2B compared with treatment subgroup 2A (P ≤ 0.006). Furthermore, Table 4 shows means of cord 25(OH)D levels were significantly increased for participants of treatment subgroup 2B (31.67 ± 7.06 ng/ml) compared with those of treatment group 2A (21.80 ± 5.67 ng/ml) (P ≤ 0.001).
| Pregnancy outcomes | Treatment subgroup 2A N = 22 (Mean ± SD) | Treatment subgroup 2 B N = 21 (Mean ± SD) | P – value |
|---|---|---|---|
| 25(OH)D (ng/ml) | 25.81 ± 5.07 | 34.67 ± 4.89* | 0.001 |
| Fasting blood sugar mg/dl | 107.55 ± 42.68 | 89.07 ± 12.96 | 0.06 |
| Insulin level (µU/ml) | 21.36 ± 17.74 | 13.71 ± 10.34 | 0.09 |
| HOMA-IR | 6.78 ± 9.99 | 2.04 ± 1.15* | 0.006 |
| Cord 25(OH)D (ng/ml) | 18.91 ± 4.15 | 27.35 ± 4.77 | 0.001 |
Table 4: Pregnancy outcomes (diabetes biomarkers and cord 25(OH)D) in both 2A and 2B treatment subgroups.
Table 5 compare pregnancy outcomes between treatment subgroups 3A and 3B. Serum 25(OH)D levels were significantly increased in treatment subgroup 3B (23.20 ± 3.95 ng/ml) compared with treatment subgroup 3A (18.06 ± 3.82 ng/ml) (P ≤ 0.001). Fasting blood sugar was significantly decreased in treatment subgroup 3B (91. 59 ± 19.65 mg/dl) compared with treatment subgroup 3A (114.20 ± 37.15 mg/dl) (P ≤ 0.008). A significantly decreased HOMA-IR was shown in treatment subgroup 3B (2.05 ± 1.35) compared with treatment subgroup 3A (6.72 ± 6.87) (P ≤ 0.006). Means of cord 25(OH)D levels were significantly increased for participants of treatment subgroup 3B (17.83 ± 4.23 ng/ml) compared with those of treatment group 3A (11.79 ± 3.62 ng/ml) (P ≤ 0.001).
| Pregnancy outcomes | Treatment subgroup 3 A N = 26 (Mean ± SD) | Treatment subgroup 3 B N = 26 (Mean ± SD) | P - value |
|---|---|---|---|
| 25(OH)D (ng/ml) | 18.06 ± 3.82 | 23.20 ± 3.95* | 0.001 |
| Fasting blood sugar mg/dl | 114.20 ± 37.15 | 91. 59 ± 19.65* | 0.008 |
| Insulin level (µU/ml) | 21.06 ± 14.75 | 17.37 ± 16.99 | 0.40 |
| HOMA-IR | 6.72 ± 6.87 | 2.05 ± 1.35* | 0.006 |
| Cord 25(OH)D (ng/ml) | 11.79 ± 3.62 | 17.83 ± 4.23* | 0.001 |
Table 5: Pregnancy outcomes (diabetes biomarkers and cord 25(OH)D) in both 3A and 3B treatment subgroups.
Table 6 shows a comparison between biomarkers before and after treatment among all treatment subgroups. It shows that serum 25(OH)D levels were significantly increased after treatment among the treatment subgroups 1B, 2B, 3A and 3B (P ≤ 0.001) when compared to levels before treatment. The highest percentage of change in serum 25(OH)D levels was shown in treatment subgroup 3B while treatment subgroup 1A shows a decreased serum 25(OH)D levels than base line levels. Fasting blood sugar shows a significantly increased serum levels among treatment subgroups 1A, 2A and 3A when compared with treatment subgroups 1B, 2B and 3B (P ≤ 0.05). Insulin levels were significantly increased in treatment subgroups 2B, 3A and 3B (P ≤ 0.03) when compared with base line levels, while HOMA-IR was significantly decreased in the same treatment subgroups (2B, 3A, and 3B) (P ≤ 0.01).
| Treatment subgroups | ||||||
|---|---|---|---|---|---|---|
| Biomarkers | Group 1A | Group 1B | Group 2A | Group 2B | Group 3A | Group 3B |
| 25(OH)D (ng/ml) (Before) | 31.86 ± 9.92 | 32.06 ± 7.86 | 23.93 ± 2.26 | 25.25 ± 5.89 | 13.89 ± 3.68 | 14.35 ± 3.99 |
| 25(OH)D (ng/ml) (After) | 29.0 ± 5.62 | 39.95 ± 8.90 | 25.81 ± 5.07 | 34.67 ± 4.89 | 18.06 ± 3.82 | 23.20 ± 3.95 |
| P – value | 0.2 | 0.001* | 0.1 | 0.001* | 0.001* | 0.001* |
| 25(OH)D (ng/ml) (Change %) | 1 % | 19.7 % | 7.8% | 27.2 % | 23.1 % | 38.1% |
| Fasting blood sugar mg/dl (Before) | 88.30 ± 14.24 | 83.40 ± 7.69 | 87.50 ± 12.57 | 88.77 ± 16.68 | 88.25 ± 11.18 | 88.87 ± 12.95 |
| Fasting blood sugar mg/dl (After) | 102.90 ± 27.73 | 80.18 ± 8.95 | 107.55 ± 42.68 | 89.07 ± 12.96 | 114.20 ± 37.15 | 91. 59 ± 19.65 |
| P – value | 0.05* | 0.4 | 0.03* | 0.9 | 0.001* | 0.4 |
| Insulin level (µU/ml) (Before) | 14.72 ± 2.37 | 13.90 ± 1.28 | 14.58 ± 2.09 | 14.79 ± 1.23 | 14.71 ± 1.86 | 14.81 ± 2.16 |
| Insulin level (µU/ml) (After) | 17.17 ± 13.43 | 13.89 ± 11.94 | 21.36 ± 17.74 | 13.71 ± 10.34 | 21.06 ± 14.75 | 17.37 ± 16.99 |
| P – value | 0.5 | 0.9 | 0.07 | 0.001* | 0.03* | 0.001* |
| HOMA-IR (Before) | 3.26 ± 1.61 | 2.89 ± 0.52 | 3.21 ± 0.92 | 3.35 ± 1.23 | 3.25 ± 0.85 | 3.32 ± 0.98 |
| HOMA-IR (After) | 4.89 ± 4.95 | 2.72 ± 2.22 | 6.78 ± 9.99 | 2.04 ± 1.15 | 6.72 ± 6.87 | 2.05 ± 1.35 |
| P – value | 0.2 | 0.8 | 0.1 | 0.001* | 0.01* | 0.001* |
Table 6: A comparison between biomarkers before and after treatment among all treatment subgroups 1A, 1B, 2A, 2B, 3A, 3B.
Discussion
Our study is one of the few clinical trials that investigate vitamin D supplementation for obese pregnant women depending serum 25(OH)D baseline levels and examine the effect of different doses of vitamin D supplementation on pregnancy outcomes. The dose response effect of vitamin D supplementation for obese pregnant women showed different outcomes depending on given dose for each category group. Higher doses of vitamin D given to treatment subgroups showed improved outcomes when compared with lower doses.
Prevalence of vitamin D deficiency among study population
The present study shows high prevalence of vitamin D deficiency and insufficiency among obese pregnant women (44% (n=52) and 36% (n=43) respectively). The remaining 20% (n=23) who showed sufficient vitamin D status were mostly those with lower BMI with around 30 kg/m2. Many studies were consistent with our findings but showed higher prevalence of vitamin D insufficiency and deficiency. An investigation of vitamin D status among Chinese adults in a population-base study it was found that 94% of study participants had vitamin D deficiency or insufficiency [11]. A randomized clinical trial of vitamin D supplementation in 162 pregnant women in United Arab Emirates reported high prevalence of vitamin D deficiency (98% of women had serum 25(OH)D levels˂20 ng/ml) [12], which was consistent with our findings. Both mentioned studies showed much higher prevalence of vitamin D deficiency in different study populations; however, small sample size in the second study might have affected results.
Effect of vitamin D supplementation on diabetes biomarkers
Many studies suggested that vitamin D levels were negatively associated with insulin resistance [13]. Results of the present study demonstrated that lower serum 25(OH)D levels were significantly associated with higher FBS levels, glucose intolerance, HOMA-IR and GDM (P ≤ 0.008). The study showed high means of baseline HOMAIR indicating a significant insulin resistance in all category groups. The higher doses of vitamin D supplementation induced insignificant negative correlation between serum 25(OH)D levels and HOMA-IR in the treatment subgroups 1A, 1B, 2B, and 3B (P˃0.05) while means of HOMA-IR were significantly decreased in treatment subgroup 2B and 3B when compared with treatment subgroup 2A and 3A respectively (P ≤ 0.006). It is obvious that increased serum 25(OH)D levels can improve insulin resistance, thus the insignificant results in treatment subgroup 1B can be attributed to several factors including small sample size, increased BMI ( ≥ 30 kg/m2), and probably that dose of vitamin D supplementation did not meet requirements and the short duration of supplementation. Consistent with our findings was reported in a cross sectional investigation of the association between serum 25(OH)D concentrations with GDM and insulin resistance in a 741 pregnant women [14]. It was founded that there was a strong correlation between serum 25(OH)D concentrations and HOMA-IR. It was suggested that vitamin D deficiency could be a confirmative sign of insulin resistance and its consequences [14]. A nested case-control study among a prospective cohort of 953 pregnant women suggested that vitamin D deficiency in early pregnancy was significantly associated with increased risk for GDM (PË‚0.001) [15]. Another cross sectional study found that vitamin D levels were inversely associated with HOMA-IR (P=0.01) and glycated hemoglobin (HbA1C) (which represents the average plasma glucose concentration) in overweight and obese individual but not in the normal weight individuals [11]. Moreover, a double blinded, randomized clinical trial performed on 81 type 2 diabetic showed that glycemic indicators (FBS and HOMAIR) were significantly improved after 8 weeks of vitamin D treatment with 50000 IU/wk compared with placebo treatment (PË‚0.001) [10].
However, the evidence of the association between vitamin D deficiency and gestational diabetes is conflicting and many studies did not show any effect on glycemia or insulin resistance. A cross sectional study of participants with metabolic syndrome showed no significant association between serum 25(OH)D concentrations and insulin action or secretion after adjustment for BMI [16]. The absence of a significant association could be attributed to the homogeneity of the study subjects that all had metabolic syndrome; thus, they had some degree of insulin resistance [16]. In another cross sectional survey of the Third National Health and Nutrition Examination Survey (1988- 1994), the results showed lack of association between vitamin D status and diabetes in non-Hispanic blacks while an inverse association was reported in non-Hispanic white and Mexican Americans [17]. This might reflect decreased sensitivity to vitamin D among non-Hispanic blacks but not-Hispanic whites or Mexican Americans. Moreover, in a randomized clinical trial, it was showed no significant effect of different doses of oral vitamin D treatment on insulin resistance in 12 week treatment duration. But the small sample size in addition to the short duration of the treatment might be limiting factors in determining the effect of vitamin D on insulin resistance [5].
Limitations
This study was limited by several determinants which made it non-reflective to the general population of pregnant women. These limitations include: small sample size, short duration of treatment, lack of nutritional assessment and absence of placebo group.
Recommendations
Based on the results of this study a routine screening of high risk pregnant women can be a major preventive measure for several pregnancy adverse outcomes. Prenatal screening of vitamin D for high risk women can be more effective in increasing chances to optimize outcomes through prevention and longer treatment duration. More randomized trials of vitamin D supplementation during pregnancy especially in obese and high risk pregnant women are needed to evaluate the potential effect of vitamin D supplementation in preventing pregnancy adverse outcomes. Larger sample size within varied population of pregnant women may add value and ascertain more representing results. Longer duration of larger randomized control trials may provide more reliable information about the optimal dose of vitamin D supplementation that induces the optimal pregnancy outcomes.
Furthermore, additional parameters and biomarkers can add a valuable support to our findings indicating conclusive results. The major obstacle was the limited financial support that could hardly cover the high cost biomarkers analysis.
Conclusion
This randomized clinical trial showed that screening for serum 25(OH)D during pregnancy and appropriate replacement, especially in patients with severe deficiency, may contribute to the prevention of gestational diabetes mellitus, improve insulin sensitivity and enhance vitamin D status. However, it is suggested that the correction of vitamin D deficiency or insufficiency through dietary supplementation can add value to the prevention of maternal and neonatal morbidity.
Acknowledgements
I would like to express my profound thanks to the Deanship of Scientific Research - Jordan University for their financial support as well as the United Pharmaceuticals who provided the supplements to support the research accomplishment.
References
- Butte NF, Lopez-Alarcon MG, Garza C (2002) Nutrient adequacy of exclusive breastfeeding for the term infant during the first six months of life. WHO, Geneva, Switzerland.
- Thacher TD, Clarke BL (2011) Vitamin D insufficiency. Mayo Clin Proc 86: 50-60.
- Thorneâ€ÂÂLyman AL, Fawzi WW (2012) Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Pediatr Perinat Epidemiol 1: 75-90.
- Gannagé-Yared MH, Chedid R, Khalife S, Azzi E, Zoghbi F, et al. (2009) Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young Middle-Eastern population. Eur J Endocrinol 160: 965-971.
- Hoseini SA, Aminorroaya A, Iraj B, Amini M (2013) The effects of oral vitamin on insulin resistance in pre-diabetic patients. J of Res in Med Sci 18: 47-51.
- Zuhur SS, Erol RS, Kuzu I, Altuntas Y (2013) The relationship between low maternal serum 25-hydroxyvitamin D levels and gestational diabetes mellitus according to the severity of 25-hydroxyvitamin D deficiency. Clinics 68: 658-664.
- Kayaniyil S, Retnakaran R, Harris SB, Vieth R, Knight JA, et al. (2011) Prospective associations of vitamin D with β-cell function and glycemia. Diabetes 60: 2947-2953.
- Oosterwerff MM, Eekhoff EM, Van Schoor NM, Boeke AJ, Nanayakkara P, et al. (2014) Effect of moderate-dose vitamin D supplementation on insulin sensitivity in vitamin D deficient non-western immigrants in the Netherlands: a randomized placebo controlled trial. Am J Clin Nutr 100: 152-160.
- Diabetes Management Guidelines (2016) Retrieved from American Diabetes Association.
- Baziar N, Jafarian K, Shadman Z, Qorbani M, Nikoo MK, et al. (2014) Effect of therapeutic dose of vitamin D on serum adiponectin and glycemia in vitamin D insufficient or deficient Type 2 diabetic patients. Iran Red Cres Med J 16: e21458.
- Lu L, Yu Z, Pan A, Hu FB, Franco OH, et al. (2009) Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes care 32: 1278-1283.
- Dawodu A, Saadi HF, Bekdache G, Javed Y, Altaye M, et al. (2013) Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. J Clin Endocrinol Metab 98: 2337-2346.
- Griz LH, Bandeira F, Gabbay MA, Dib SA, Carvalho EF (2014) Vitamin D and diabetes mellitus: an update – 2013. Arq Bras Endocrinol Metab 58: 1-8.
- Maghbooli Z, Hosseinâ€ÂÂnezhad A, Karimi F, Shafaei AR, Larijani B (2008) Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes Metab Res Rev 24: 27-32.
- Zhang C, Qiu C, Hu FB, David RM, Van Dam RM, et al. (2008) Maternal plasma 25-hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PloS ONE 3: e3753.
- Gulseth HL, Gjelstad IM, Tierney AC, Lovegrove JA, Defoort C, et al. (2010) Serum vitamin D concentration does not predict insulin action and secretion in European subjects with the metabolic syndrome. Diabetic Care 33: 923-925.
- Scragg R, Sowers M, Bell C (2004) Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes care 27: 2813-2818.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi