Market Analysis, J Pharm Sci Emerg Drugs Vol: 7 Issue: 2
Global presence of your research at Biologics Meet 2020 scheduled during Nov 16-17, 2020 at Kyoto, Japan
Gerald Tan
Tan Tockseng Hospital, Singapore,E-mail: mail@geraldtan.com
Keywords: pharmaceutical drugs
Biologics Meet 2020 describes recent developments in the marketing and production of pharmaceutical drugs and contract manufacturing. A comprehensive knowledge of a scientific discipline that describes the effects of Biosimilars drug marketing and Biologics meet 2020 is now exploring the scope of Biosimilars drug marketing in the industry. Biologics meet 2020 provides detailed market, technology and industry analysis to help readers quantify and qualify the generic prescription market. Significant trends are identified and sales forecasts by product category and major national markets are based on industry sources and an assessment of the regulatory environment, health policies, demographics and other factors directly affecting the generic market. The wider economic environment is also taken into account.
Drug discovery volumes continue to rise in the U.S. and chemical market expected to contract this year—As a result, chemical industry capital spending in the U.S. surged 12.1% in 2014 and gained 21.0% in 2015, reaching $ 43.58 billion and accounting for more than one-half of total construction spending by the manufacturing sector. The association representing US-based chemical producers said that US chemical production (excluding pharmaceuticals) is expected to realize the overall growth of 1.6% in 2016, followed by 4.1% growth next year, and 5.0% in 2018. Average annual gains of over 8% per year in U.S. Chemical industry capital spending are expected through 2018 with only a minor slowdown in subsequent growth expected. By 2021, ACC expects capital spending to reach $70 billion, contributing to four consecutive years of job growth in the industry. American chemistry revenues will exceed $1.0 trillion by 2020
Pharmacy Council stated that more than 275 new chemical production projects had been announced since 2010 with a total value of more than $170 billion, with a full 49% already complete or under construction; 61% of these are the foreign direct investment. By 2021, U.S. capital spending by the chemical industry will reach $65 billion—more than triple the level of spending at the start of this prolonged cycle in 2010. The trade surplus in chemicals (excluding pharmaceuticals) will grow to $36 billion this year as exports rise by 2% to $132 billion and imports hold steady at $96 billion. Two-way trade between the U.S. and its foreign partners will reach $227 billion this year and will grow steadily over the coming years.
The popularity of Formulation and Drug Discovery has increased significantly in recent years. The Drug Delivery Technology market is expected to reach USD 1,504.7 Billion by 2020 from USD 1,048.1 Billion in 2015, growing at a CAGR of 7.5% from 2015 to 2020. Drug delivery technology market offers a promising approach for the delivery of various kinds of drugs that have different molecular formulation. Drug delivery technology is aimed at maximizing the drug delivery at the targeted site so as to increase the efficiency of drug and proposing improved patient compliance.
Importance & Scope:
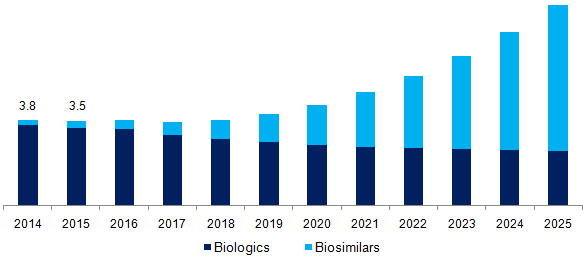
Biotechnology drugs will overtake small molecule pharma drugs in coming decades. As per EvaluatePharma report, use of biotech drugs will continue to rise, contributing to 50 per cent of the top 100 pharma sales by 2022. The uptake of biologics is expected to continue as novel biologic blockbusters keep entering the pharmaceutical market. The penetration of biotech products is set to increase from a 24 per cent market share in 2015 to 29 per cent in 2022. According to a latest forecast, in 2022, 50 per cent of the value of the top 100 products will come from biologics as established chemical products drop off the patent cliff and new breakthrough biologics get approved.
Biosimilars:
The US FDA defines biosimilars “a biosimilar is a biological product that is highly similar to a US-licensed reference biological product not withstanding minor differences in clinically inactive components, and for which there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity and potency of the product”.
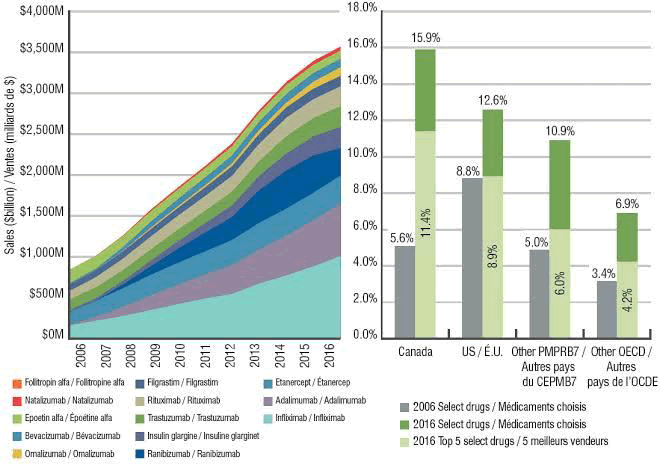
Taking a leaf from small molecules, in the US alone, generics accounted for 86 per cent of all dispensed retail prescriptions in 2013. This saved their economy around $200 billion. Cost savings, affordability and access becomes tantamount for biologics, being pricy in the first place. While many biologics are addressing significant unmet medical needs, they are undoubtedly expensive and unaffordable to many. Some of these therapies cost upwards of $100,000 per treatment course on an annualized basis. There is no doubt that biosimilars bear the potential to rationalize spending on drugs in developed economies and provide access to this critical economic driver, in developing economies.
Roundtables: Provide a platform where industry professionals meet with academic experts.
More than 50 international organizations and pavilions will exhibit at Biologics meet 2020 pharma conference. Exhibitors will include equipment manufacturers and suppliers, system suppliers, finance and investment companies, research and development companies, developers projects, trade associations and government agencies.
In addition to products and services, you'll have access to valuable content, including keynote presentations, product demonstrations, and training sessions offered by industry leaders today.
The Biologics meet 2020 brings together everything you need, under one roof, to save you time and money. This is the event not to be missed!
A two-day rally that examines future market models, creative business methodologies and open doors to the development of moderate medicines. Pioneers in the pharmaceutical industry want substance and roundtable reviews to remain for systems administration and air. This meeting is officially the largest non-proprietary key meeting of the company and will provide members with a comprehensive overview of the business methodology for moderate medications.
Get your image before senior chiefs
Break into the lucrative Generic Medicines industry
Position your organization as an industry pioneer
Make deals leads and convey an arrival on venture
Connect with your objective market in the locale.
Target Audience
• Directors, CEO’s of Organizations
• Business Development Managers
• Chief Scientific Officers
• R&D Researchers from Biosimilar and Biologics Industries
• Professors, Associate Professors, Assistant Professors
• PhD Scholars
• Patent Attorneys
• Intellectual Property Attorneys
• Investment Analysts
• Association, Association presidents and professionals
• Noble laureates in Health Care and Medicine
• Bio instruments Professionals
• Bio-informatics Professionals
• Software development companies
• Research Institutes and members
• Supply Chain companies
• Manufacturing Companies
• CRO and DATA management Companies
• Training Institutes
• Business Entrepreneurs
Related Companies/Industries
• A Menarini Asia-Pacific Holdings Pte Ltd,
• Ab Sciex (Distribution)
• Abbott Laboratories (S) Pte Ltd,
• Abbott Mfg Singapore Pte Ltd,
• Abbvie Pte Ltd,
• Acyx Enterprise
• Advance Healthcare Pte Ltd,
• Advanced Medi Mart,
• Agila Specialties Global Pte Ltd,
• Alcon Singapore Mfg Pte Ltd,
• Allergen Singapore Pte Ltd,
• Apd Pharmaceutical Mfg Pte Ltd,
• Apex Pharma Marketing Pte Ltd,
• Apotheca Marketing Pte Ltd,
• Aslant Pharmaceuticals Pte Ltd,
• Astonix Life Science (S) Pte Ltd,
• Astrazeneca Singapore Pte Ltd,
• Atlantic Pharmaceutical (S) Pte Ltd,
• Aurum Medicare Pte Ltd,
• Aventis Pharma Mfg Pte Ltd,
• Ayuryoga Clinic of Ayurveda & Yoga Pte Ltd,
• Bago Laboratories Pte Ltd,
• Beacons Pharmaceuticals Pte Ltd,
• Beecham Pharmaceuticals (Pte) Ltd,
Related Associations and Societies
• International Pharmaceutical Federation (FIP)
• International Pharmaceutical Students' Federation (IPSF)
• European Association of Employed Community Pharmacists in Europe (EPhEU)
• Pharmaceutical Group of the European Union (PGEU)
• Australian College of Pharmacy ......
• Pharmaceutical Society of Australia
• The Pharmacy Guild of Australia
• The Society of Hospital Pharmacists of Australia
• Canadian Pharmacists Association
• Canadian Society of Hospital Pharmacists
• Ontario Pharmacists Association
-
World Congress on Biologics and Biosimilars
Kyoto, Japan
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 