Research Article, J Pharm Sci Emerg Drugs Vol: 5 Issue: 1
Green Method for Quantification of Sodium Cefotaxime in Lyophilized Powder by Infrared Spectroscopy
Lívia Paganini Consortti* and Hérida Regina Nunes Salgado
Department of Drugs and Pharmaceuticals, School of Pharmaceutical Sciences, São Paulo State University (UNESP), Araraquara, Brazil
*Corresponding Author : Lívia Paganini Consortti
Department of Drugs and Medicines, School of Pharmaceutical Science, Univ. Estadual Paulista - UNESP, CEP: 14801-902, Araraquara, SP, Brazil
Tel: +551633016880
Fax: +551633220073
E-mail: liviaconsortti@gmail.com
Received: January 04, 2017 Accepted: January 18, 2017 Published: January 24, 2017
Citation: Consortti LP, Salgado HRN (2017) Green Method for Quantification of Sodium Cefotaxime in Lyophilized Powder by Infrared Spectroscopy. J Pharm Sci Emerg Drugs 5:1. doi:10.4172/2380-9477.1000118
Abstract
The bacterial resistance to antimicrobial is a growing difficulty found in the public health, so, all the steps of production need a strict control to minimize this problem. The quality control is one of the stages that is used in pharmaceutical industry to ensure the effectiveness of treatment and patient safety. One of antimicrobial drugs is Cefotaxime, used in treatment of meningitis and septicaemia by Gram-negatives. In the literature, there are many methods for quantification of the drug, but all of them have a great environmental impact. Therefore with the awareness about the adoption of green procedures in pharmaceutical industry, it is necessary to find new methods with reduction of waste generation and use of toxic reagents and solvents. This paper validated a new green method for quantification of sodium cefotaxime by infrared spectroscopy according to ICH (International Harmonised Tripartite Guideline). The quantification was developed using the absorbance relative to the band of the carbonyl in the β-lactam ring. The method presented suitable linearity, precision, accuracy and robustness in the range of 0.4 to 0.9 mg/pellet and capacity of quantify the cefotaxime in the pharmaceutical product with confidence as a green alternative method.
Keywords: Cefotaxime; Infrared spectroscopy; Quality control; Validation; Quantification; Green chemistry
Introduction
Antimicrobials are part of a class of very important drugs in the world for the high mortality rate for bacterial infections. As an aggravating, bacterial resistance is growing and extending to different environments, not only to the hospital [1,2]. Therefore, all steps of production of this class of drugs, and treatment should be monitored to ensure the effectiveness of treatment and minimize the growth of bacterial resistance [3,4].
Among the activities included in the adequate production of an antimicrobial agent is quality control. This step is crucial for checking the suitability specifications of pharmaceutical raw materials before production of pharmaceutical products, the product in manufacturing process and the finished product. For checking these characteristics, analytical methods are used for the identification and quantification of feedstock, pharmaceutical products and impurities [3,4]. The most used techniques for analysis of drugs and other pharmaceutical products are the HPLC (high performance liquid chromatography) and spectrophotometry in UV-Vis (ultraviolet-visible).
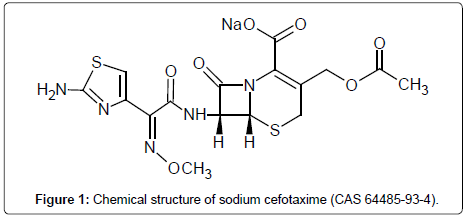
One of antimicrobial drugs is sodium cefotaxime (Figure 1), a semisynthetic molecule obtained from a fermentation process. It is a third generation cephalosporin primarily used in the treatment of meningitis and septicemia caused by Gram-negative microorganisms for achieving adequate concentrations to their action in the central nervous system [5,6].
Due the bacterial resistance growing to the antibiotics, it is necessary to implement an appropriate quality control, but linking to the green analytical chemistry apllying it’s principles to minimize the environmental impact and the operator safety in pharmaceutical industry and in the academic. The awareness about environmental impact of industrial and research procedures is taking increasing dimensions, what is evidenced by the significant increase of publications in the area [7].
In the literature is mainly found analytical methods for quantification of sodium cefotaxime by HPLC [8-12] and spectrophotometry in UV-Vis [13-17]. However, the methods reported use large quantities of toxic solvents and have high waste generation. With the increasing awareness about the need of the use of environmentally friendly procedures in the pharmaceutical industry in recent years, the adoption of green alternative analytical methods in the routine of drug quality control and pharmaceutical products has great relevance [18,19].
The IR technique is widely used for the identification of drugs and medicines in pharmaceutical industry, but more recent studies demonstrate the technique’s ability to quantify molecules in appropriate and simple manner, low cost and low generation of toxic waste when compared to the techniques of HPLC and UV-Vis [20-30].
Infrared spectroscopy technique have some advantages, as reduced waste generation, execution simplicity, low analysis time and possibility of the analysis of insoluble material in the commonly used solvents for preparing solutions for another analysis techniques [22]. A large number of methods with high environmental impact in the literature for sodium cefotaxime is described. Due to these factors, we sought to employ the infrared technique for development and validation of a new analytical method for quantification of sodium cefotaxime in lyophilized powder aimed at contributing to the drug quality control by introducing an environmentally friendly method.
Materials and Method
Chemicals and reagents
Sodium cefotaxime reference standard (RS) was commercially purchased from Sequoia Research Products (purity content of 98.0%). The sodium cefotaxime lyophilized powder used was CetazimaTM, commercialized by Novafarma Pharmaceutical Industry (Anápolis, Goiás, Brazil). Each flask contains 524 mg of sodium cefotaxime, equivalent to 500 mg of base cefotaxime. Adjuvants are not present in the pharmaceutical.
Potassium bromide used for pellets preparation (Synth® - Diadema, São Paulo, Brazil and Dinâmica® - Diadema, São Paulo, Brazil) was analytical grade. To obtain the pellets, potassium bromide was pulverized in an agate mortar to obtain a fine and homogeneous powder and dried to constant weight at 105°C.
Apparatus
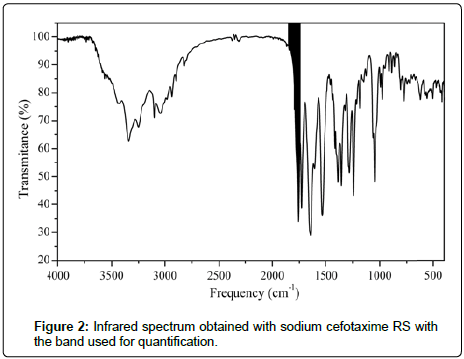
An infrared spectrometer Shimadzu® with Fourier transform, model IR Prestige-21 (Kyoto, Japan) was used to obtain the spectrums in the region of medium infrared (400 to 4000 cm-1), with resolution of 2 cm-1. The quantification was developed with the software IR Solution by the analysis of the absorbance relative to the peak height in the region from 1825 to 1740 cm-1 in the sodium cefotaxime spectrum (Figure 2), which refers to the stretching of the double bond between carbon and oxygen (carbonyl) of the lactam group found in the molecule sodium cefotaxime.
Experimental
Preparation of the pellets
In manufacture of the pellets, the sample (RS sodium cefotaxime and/or sodium cefotaxime lyophilized powder) was diluted with potassium bromide in a ratio of 1:100 (w/w) and certain amount of this diluted potassium bromide was homogenized with sufficient amount of potassium bromide to obtain the pellets with a final weight of 200 mg in the proper concentration. The powder mixture was then submitted to a pressure of 80 kN for 10 minutes resulting in translucid pellets.
Method validation
The method validation was developed according to ICH Q2 (R1). The parameters evaluated were: linearity, precision, accuracy, limit of detection, limit of quantification and robustness [31].
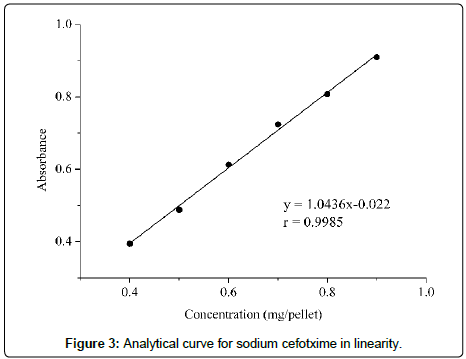
Linearity: The curve was constructed in the interval of concentrations of 0.4-0.9 mg/pellets. Amounts equivalent to 0.4, 0.5, 0.6, 0.7, 0.8 and 0.9 mg of the diluted potassium bromide with cefotaxime (prepared in item Preparation of the pellets) were homogenized with enough bromide potassium to obtain 200 mg pellets. After constructing the graph of the linear regression with the absorbance versus concentration, it was performed the statistical analysis. The significance of regression was evaluated by analysis of variance (ANOVA), considering a level of significance of 5%, the correlation coefficient was calculated and the residues graph was plotted. The linearity was carried out in three days [31].
Precision: The precision was evaluated in two levels: repeatability and intermediate precision. The pellets were prepared with the sodium cefotaxime lyophilized powder in three concentrations (0.4, 0.7 and 0.9 mg/pellet). For repeatability, were prepared 3 pellets for each concentration, with the same analyst and in the same equipment. Intermediated precision was carried out the same analytical conditions to repeatability, but in a different day and with another analyst. The analysis considered for this parameter was the relative standard deviation, in percentage, for the replicates for each analyst and between two analysts for the three concentrations [31].
Accuracy: Accuracy was evaluated according to the recovery test, in which the RS cefotaxime (CTX) was added to known amounts of lyophilized powder in three concentration levels (low, medium and high), considering the linear range of the method (Table 1). The assay was performed in triplicate and is presented in Table 1 [31].
| Pellet | CTX lyophilized powder1 (mg) | CTX RS1 (mg) | Potassium bromide2 (mg) | Final concentration (mg/pellet) |
|---|---|---|---|---|
| Lyophilized powder | 40 | - | 160 | 0.40 |
| R1 | 40 | 16 | 144 | 0.56 |
| R2 | 40 | 30 | 130 | 0.70 |
| R3 | 40 | 44 | 116 | 0.84 |
| RS | - | 40 | 160 | 0.40 |
Table 1: Preparation of potassium bromide pellets for recovery test.
The recovery, in percent, was calculated by means of equation 1, according to established by AOAC (Association of Official Analytical Chemists) (2002) [32].
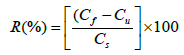 (1)
(1)
Where:
R(%): recuperation (percentage);
Cf: concentration of sodium cefotaxime in lyophilized powder added with cefotaxime RS (mg/pellet);
Cu: concentration of sodium cefotaxime in lyophilized powder (mg/pellet);
Cs: Theoretical concentration of sodium cefotaxime RS added (mg/pellet).
Detection limit and quantitation limit: The detection limit (DL) and quantitation limit (QL) were estimated by equations 2 and 3, respectively. These parameters are not required to this validation, because the intended porpouse of the method is develop an assay test, but were calculated as complement [31].
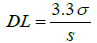 (2)
(2)
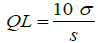 (3)
(3)
Where:
σ = standard deviation of response;
S = slope of calibration curve.
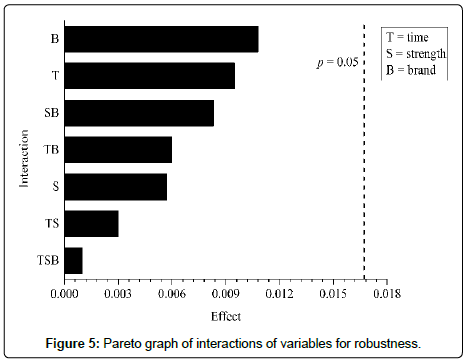
Robustness: The robustness was evaluated according to the factorial design 23. In this experiment we sought to evaluate the influence of small variations of three factors in the analysis conditions in two levels. The 3 factors and their variations are described in Table 2. From the establishment of the parameters to be evaluated, the test was developed following the planning matrix for factorial design 23. Were prepared 16 pellets containing sodium cefotaxime lyophilized powder at the working concentration (0.7 mg/pellet), two from each of the 8 runs [33,34].
| Factors | (-) | (+) |
|---|---|---|
| 1: Compression time (min) | 9 | 11 |
| 2: Compression strength (kN) | 76 | 84 |
| 3: Potassium bromide brand | Synth | Dinamica |
Table 2: Factors and variations for robustness by factorial design 23.
Interference of change of each factor, and the combination of factors in the analysis was statistically evaluated by ANOVA, calculated with the aid of the software Assistat 7.7 beta and using the t test, assessing the significance of the effects (contrast coefficients) demonstrated in the construction of Pareto chart. Effects were calculated from the average absorbance of each test, then the effects were calculated by subtracting the average of the absorbance values the higher level (+) of the average absorbance values of the lower level (-). The statistic analysis were avaliated in a significance level of 5% [33,34].
Determination of sodium cefotaxime in the lyophilized powder
After method validation, the assay for sodium cefotaxime in the final product was determined.
Pellets preparation: The sodium cefotaxime RS and lyophilized powder pellets were prepared in the working concentration, weighting up diluted drug equivalent to 0.7 mg of sodium cefotaxime (70 mg of drug diluted in potassium bromide 1:100 w/w) and 130 mg of potassium bromide. For the manufacture of pellets containing sodium lyophilized powder cefotaxime, first was prepared a pool with the contents of twenty flasks of the product, and then was prepared diluted with potassium bromide. The determinations were performed in triplicate.
Calculations: The content of sodium cefotaxime in the lyophilized powder was calculated in mg/pellet, according to Equation 4, and in percentage, according to Equation 5.
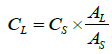 (4)
(4)
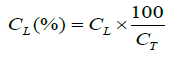 (5)
(5)
Where:
CL: concentration of sodium cefotaxime in lyophilized powder pellet (mg/pellet);
CS: concentration of sodium cefotaxime in RS pellet (mg/pellet);
AL: absorvance of sodium cefotaxime in lyophilized powder pellet;
AS: absorvance of sodium cefotaxime in RS pellet;
CL: concentration of sodium cefotaxime in lyophilized powder (in percentage);
CT: theoretical concentration of sodium cefotaxime in lyophilized powder in the pellet (mg/pellet).
Results and Discussions
First, were obtainet the sodium cefotaxime RS and sodium cefotaxime lyophilized powder spectrums, and after were overlaied to identification of the drug in the pharmaceutical product? The presence of characteristic bands with similar frequency values and intensities confirms the identity of the sodium cefotaxime in the lyophilized powder, which can also be observed by comparison with reference spectra of Japanese Pharmacopeia [35].
The infrared spectrum presented caracteristic absorption bands such as 3431 and 3344 cm-1 (stretch of the binding N-H of primare amine), 2935 cm-1 (stretch of the binding C-H of Csp3), 1759 cm-1 (stretch of the binding C=O of lactam), 1730 cm-1 (stretch of the binding C=O of ester), 1647 cm-1 (stretch of the binding C=O of amide), 1608 cm-1 (asymmetrical stretch of binding C=O of carboxyl group), 1386 and 1354 cm-1 (symmetrical stretch of binding C=O of carboxyl group and folding of binding C-H of methyls), 1242 cm-1 (stretch of the binding C-N of β-lactam ring) and 1047 cm-1 (stretch of the binding C-O of ester), and according with the spectrum in Figure 2 and the reference, the frequency values of the sodium cefotaxime RS spectrum bands are consistent with those found in the literature, which emphasizes the presence of the molecule in lyophilized powder [20,21].
The spectral region chosen for the quantification considered the band belonging to the selective grouping for the class of antimicrobials to which belongs the drug, the cephalosporins [6].
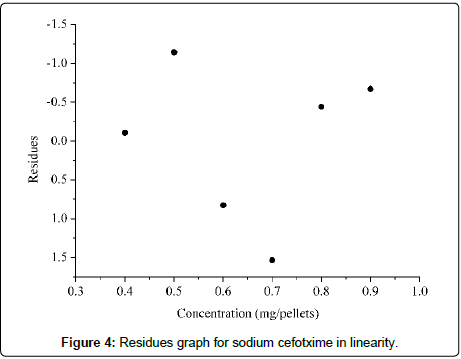
The calibration curve was obtained by plotting a graph of absorbance versus concentration in the range of 0.4 to 0.9 mg/ pellet (Figure 3). The value of 0.9985 of the correlation coefficient demonstrates the strong association between the two variables. The analysis of variance confirmed that the regression is statistically significant, since the P-value obtained for the evaluation of the angular coefficient was less than 0.05 (2.90×10-6) (significance level of 5%). The presence of statistical regression is also confirmed by the fact that the straight line pass through the origin, since the P-value obtained for the evaluation of the intersection is equal to 0.300, at a 5% significance level. The residues graph, presented in Figure 4, shows adequate distribution, confirming the linearity of the method in the concentration range evaluated [30,36].
The precision evaluated at two levels (repeatability and intermediate) showed compliance of the method for introducing RSD values lower than 5% (Table 3).
| C1 | Analyst 1 | Analyst 2 | RSD4 (%) | ||
|---|---|---|---|---|---|
| Average absorbance ± SD2 | RSD3 (%) | Average absorbance ± SD2 | RSD3 (%) | ||
| 0.4 | 0.422 ± 0.0026 | 0.63 | 0.400 ± 0.0138 | 3.45 | 3.73 |
| 0.7 | 0.734 ± 0.0231 | 3.14 | 0.717 ± 0.0097 | 1.35 | 1.62 |
| 0.9 | 0.897 ± 0.0130 | 1.45 | 0.935 ± 0.0053 | 0.57 | 2.93 |
Table 3: Values obtained with precision by repeatability and intermediate precision.
The method presented is accurate for recovery values for the three levels are close to 100%. The average recovery was obtained equal to 100.45% (Table 4).
| CTX RS added (mg) | CTX RS found (mg) | Recovery (%) | RSD1 (%) | Average recovery (%) | |
|---|---|---|---|---|---|
| R1 | 0.16 | 0.158 | 98.48 | 0.74 | |
| R2 | 0.30 | 0.302 | 100.79 | 1.19 | 100.45 |
| R3 | 0.44 | 0.449 | 102.08 | 1.91 |
Table 4: Values of accuracy by recovery.
In the robustness parameter, after application of the variance analysis, it was observed that the changes considered for the evaluated parameters did not influenced in the absorbance values, since P-value was greater than 0.05 for all of the interactions: 0.0949 (factor 1), 0.2849 (factor 2), 0.5664 (factor 3), 0.0644 (interaction factors 1 and 2), 0.2660 (interaction factors 1 and 3), 0.1387 (interaction factors 2 and 3) and 0.8469 (interaction factors 1, 2 and 3) [32,33].
By observing the effects values arranged in the Pareto chart (Figure 5), is noted that there is no interaction with absorbance values statistical significant for a P-value of 0.05, in other words, none of the altered factors interfere significantly in the equipment response. Despite the demonstration of the method robustness, it is important to emphasize that the change of potassium bromide brand showed larger variation in the results [32,33].
Validation of the method by infrared absorption spectroscopy showed that it is suitable and reliable for the quantification of cefotaxime sodium in the lyophilized powder. Furthermore, the sample preparation is simple and fast, the technique can be used for insoluble or poorly soluble and unstable drugs in solvents used to prepare solutions for analysis by another techniques, such as high-performance liquid chromatography or ultraviolet spectrophotometry [21].
The developed and validated method has the great advantage of having a low waste generation, since it uses low amounts of potassium bromide and drug. Quantification of the drug by spectroscopy infrared does not require the use of organic solvents or toxic reagents both for the analyst and the environment used in the methods by other techniques, such as ultraviolet spectrophotometry found in the literature [14,16,37,38] and it complies with a large part of the green chemistry principles [7].
After validation of the analytical method, it was determined the content of sodium cefotaxime in the lyophilized powder and the average score was 99.28%, therefore, the pharmaceutical product is in accordance with the specification set by monograph found in The United States Pharmacopeia (2014), from 90.0 to 115.0% [35].
Conclusion
The validation demonstrated that the developed method is suitable and reliable for the quantification of sodium cefotaxime in the lyophilized powder. Analytical procedures are simple, and the technique can be used for unstable drugs in aqueous solutions or other solvents, insoluble or low solubility drugs in solvents used to prepare samples for analysis by other techniques, such as high-performance liquid chromatography or ultraviolet spectrophotometry [21].
The method also has the great advantage of having a low waste generation, since it uses low amounts of potassium bromide. The quantification of the drug by infrared spectroscopy also does not require the use of organic solvents or toxic reagents, for the analyst and the environment, used in the methods by other techniques, such as ultraviolet spectrophotometry found in the literature. This characteristics of the technique can qualify the developed and validated method as green [13,15,37,38,39].
Acknowledgements
This work was supported by PACD-FCFAr-UNESP (Araraquara, Brazil), FAPESP (São Paulo, Brazil), and CNPq (Brasília, Brazil). L.P. Consortti was funded by CAPES (Brasília, Brazil) and H.R.N. Salgado was funded by CNPq (Brasília, Brazil).
References
- Guimarães DO, Momesso LS, Pupo MT (2010) Antibióticos: Importância terapêutica e perspectivas para a descoberta e desenvolvimento de novos agentes. Quím. Nova 33: 667-679.
- Brito MA, Cordeiro BC (2012) Necessidade de novos antibióticos. J Bras Pat Med Lab 48: 247-249.
- Haleem RM, Salem MY, Fatahallah FA, Abdelfattah LE (2013) Quality in the pharmaceutical industry - A literature review. Saudi Pharm J 23: 463-469.
- Agência Nacional de Vigilância Sanitária. Resolução (2010) RDC nº 17 de 16 de abril de 2010. Provides on Pharmaceutical Good Manufacturing Practices. Diário Oficial da União Brasília.
- Fernandes R Amador P Prudêncio C (2013) β-Lactams: chemical structure mode of action and mechanisms of resistance. Rev Med Microb 24: 7-17.
- Patrick G L (2009) An Introduction to Medicinal Chemistry (4th Edtn.) Oxford University Press New York USA.
- Galuszka A, Migaszewski Z, Namiesnik J (2013) The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. Trends Anal Che 50: 78-84.
- Iqbal MS, Bahari MB, Darwis Y, Iqbal MZ, Hayat A et al. (2013) An RP-HPLC-UV Method with SPE for cefotaxime in all-in-one total parenteral nutritional admixtures: application to stability studies. J AOAC Int 2: 290-294.
- Saranya CHL, Thejaswini JC, Gurupadayya BM, Sruthi BYK (2014) Simultaneous determination of sodium cefotaxime and paracetamol by LC-MS. IOSR J Pharm 4: 12-18.
- Wang P, Yuan T, Hu J, Tan Y (2011) Determination of cephalosporin antibiotics in water samples by optimised solid phase extraction and high performance liquid chromatography with ultraviolet detector. Int J Environ Anal Chem 91: 1267-1281.
- Manda P, Hargett JK, Vaka SR, Repka MA, Murthy SN (2011) Delivery of cefotaxime to the brain via intranasal administration. Drug Dev Ind Pharm 37: 1306-1310.
- Zhu T, Row KH (2009) Extraction and determination of cefazolin sodium and sodium cefotaxime in human urine with a weak ion exchange monolithic column. J Liq Chrom Rel Technol 32: 1423-1433.
- Aswani KCH, Gurupadayya BM, Navya SS (2011) Determination and validation of cefadroxil ceftriaxone and cefotaxime by using n-bromosuccinamide in human plasma and pharmaceutical dosage form. Int J Res Pharm Sci 2: 206-212.
- Sayed RA, Hassan WS, El-Mammli MY, Shalabya A (2012) A new extractive spectrophotometric method for the determination of gatifloxacin and sodium cefotaxime in pure and pharmaceutical dosage forms. Oriental J Chem 28: 639-650.
- Rageh AH, El-Shaboury SR, Saleh GA, Mohamed FA (2010) Spectophotometric method for determination of certain cephalosporins using 4-chloro-7-nitrobenzo-2-oxa-13-diazole (NBD-Cl). Nat Sci 2: 828-840.
- Sayed RA (2013) Spectrophotometric method for the determination of sodium cefotaxime and cefoperazone sodium in pure and pharmaceutical dosage forms. Am Chem Sci J 3: 514-525.
- Bagheri GHA, Rad YA, Rezvani M, Roshanzamir S (2012) Spectrophotometric complexation of cephalosporins with palladium (II) chloride in aqueous and non-aqueous solventes. Spectrochim Acta A Mol Biomol Spectrosc 89: 317-321.
- Armenta S, Garrigues M, Guardia M (2008) Green analytical chemistry. Trends Anal Chem 1380: 497-511.
- Welch CJ, Wu N, Biba M, Hartman R, Brkovic T, et al. (2010) Greening analytical chromatography. Trends Anal Chem 29: 667-680.
- Pavia DL, Lampman GM, Kriz GS, Vyvyan JR (2008) Introduction to spectroscopy (4th Edtn.). Cengage Learning: Belmont.
- Silverstein RM, Bassler CG, Morril TC (2005) Spectrometric identification of organic compounds (7th Edtn.). J Wiley: Hoboken.
- Moreno AH, Salgado HRN (2012) Development and validation of the quantitative analysis of ceftazidime in powder for injection by infrared spectroscopy. Phys Chem 2: 6-11.
- Tótoli EG, Salgado HRN (2012) Development and validation of the quantitative analysis of ampicillin sodium in powder for injection by Fourier-transform infrared spectroscopy (FT-IR). Phys Chem 2: 103-108.
- Vieira DCM, Ricarte PC, Salgado HRN (2012) Development and validation of the quantitative analysis of cefuroxime sodium in powder for injection by infrared spectroscopy. Adv Anal Chem 2: 80-87.
- Ali M, Sherazi STH, Mahesar SA (2014) Quantification of erythromycin in pharmaceutical formulation by transmisson Fourier transform infrared spectroscopy. Arabian J Chem 7: 1104-1109.
- Kogawa AC, Salgado HRN (2012) Development and validation of infrared spectroscopy method for the determination of darunavir in tablets. Phys Chem 3: 1-6.
- Corrêa JCR, Salgado HRN (2014) A plataform for designing quantitative infrared spectrophotometric method for drugs and pharmaceuticals analysis: a rediscover for an ecological and safer technique in the routine quality control laboratories. World J Pharm Pharm Sci 3: 2056-2059.
- Mallah MA, Sherazi STH, Bhanger MI, Mahesar SA, Bajeer MA () A rapid Fourier-transform infrared (FTIR) spectroscopic method for direct quantification of paracetamol content in solid pharmaceutical formulations. Spectrochim Acta A Mol Biomol Spectrosc141: 64-70.
- Kogawa AC, Mello NP, Salgado HRN (2016) Quantification of doxycycline in raw material by an eco-friendly method of infrared spectroscopy. Pharm Anal Acta 7: 463-466.
- Natori JSH, Tótoli EG, Salgado HRN (2016) Development and validation of a green analytical method for determination of norfloxacin in raw material by Fourier-Transform Infrared Spectrophotometry (FT-IR). JAOAC Int.
- International Harmonised Tripartite Guideline (ICH) (2005) Validation of analytical procedures: text and methodology Q2 (R1). Commission of the European Communities Geneva.
- Association of Official Analytical Chemists (2002) Official methods of analysis (17th Edtn.). AOAC: Gaithesburg.
- Montgomery DC (2009) Design and Analysis of Experiments (7th Edtn.), John Wiley & Sons: New York, USA.
- Neto BB, Scarminio IS, Bruns RE (2011) Como fazer experimentos (4th Edtn.), Bookman: Porto Alegra.
- Society of Japanese Pharmacopoeia (2011) Japanese Pharmacopoeia (16th Edtn.), Tokyo.
- The United States Pharmacopeia (2014) The Nacional Formulary (NF 34) (39th Edtn.). United States Pharmacopoeia Convention: Rockville, USA.
- Montgomery DC, Peck EA, Vinning GG (2006) Introduction to linear regression analysis (4th Edtn.). John Wiley & Sons: New York, USA
- Fan B, Gengb M, Wangb Y, Lai Q (2013) Spectrophotometric determination of cefotaxime by Using Sodium 12-Naphthoquinone-4-Sulfonate. J Anal Chem 68: 965-968.
- Omar AM, Abdelmageed OH, Attia TZ (2009) Kinetic spectrophotometric determination of certain cephalosporins in pharmaceutical formulations. Int J Anal Chem 2: 1-12.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi