Research Article, J Pharm Sci Emerg Drugs Vol: 5 Issue: 1
Hydroxypropyl-Beta-Cyclodextrin as Cryoprotectant in Nanoparticles Prepared By Nano-Spray Drying Technique
Amr Maged1*, Azza A Mahmoud1,2 and Mahmoud M Ghorab3
1Department of Pharmaceutics & Pharmaceutical Technology, Future University, Cairo, Egypt
2Department of Pharmaceutical Technology, National Research Center, Dokki, Cairo, Egypt
3Department of Pharmaceutics, Faculty of Pharmacy, Cairo University, Cairo, Egypt
*Corresponding Author : Amr Maged
Department of Pharmaceutics & Pharmaceutical Technology, Future University in Egypt, End of 90th St., Fifth Settlement, New Cairo, Egypt
Tel: +201005849238
E-mail: amr.maged@fue.edu.eg
Received: July 26, 2017 Accepted: August 04, 2017 Published: August 11, 2017
Citation: Maged A, Mahmoud AA, Ghorab MM (2017) Hydroxypropyl-Beta-Cyclodextrin as Cryoprotectant in Nanoparticles Prepared By Nano-Spray Drying Technique. J Pharm Sci Emerg Drugs 5:1 doi: 10.4172/2380-9477.1000121
Abstract
Nano-spray dryer is advanced instrument to produce a stable and spherical nanoparticles with high yield. In this study, econazole nitrate nanoparticles were formulated by nano-spray dryer using 1:1, 1:2 and 1:3 weight ratios of drug to hydroxypropyl-betacyclodextrin and stabilizer. The prepared samples were sprayed through nozzle size of 7.0 μm using 95ºC and 45ºC as inlet temperature and outlet temperatures, respectively. The prepared nanoparticles were evaluated for process yield and percent drug loading. Furthermore, the drug nanoparticles were dispersed in isotonic buffer solution and examined for drug release and their stability at room temperature. The spray dried particles were in the nano-range (148 to 294 nm) and their yield values ranged between 79.1 and 84.9 %. Increasing weight ratio of drug to hydroxypropylbeta- cyclodextrin to 1:2 and 1:3 showed increases in percent drug release compared to formulation containing 1:1 weight ratio of drug to hydroxypropyl-beta-cyclodextrin. On the other hand, the prepared econazole nitrate nanosuspension containing 1:1 weight ratio of drug to hydroxypropyl-beta-cyclodextrin revealed best stability study during storage period at room temperature compared to other formulations. As a result of in-vitro drug release and stability studies, the optimum weight ratio of 1:1, drug to hydroxylpropylbeta- cyclodextrin was chosen as a best weight ratio duo to its good balance between drug release and stability of drug loaded nanoparticles.
Keywords: Nano-spray dryer; Nanoparticles; Hydroxypropyl-β-cyclodextrin; Stability
Introduction
Nano-spray drying is a new method to achieve drug particles in nanosize with high yield and low cost. The nano-spray dryer consists of inlet tube for sample entry and outlet one to recovery the excess sample to be used again without causing any loss in sample volume, and it also consists of drying chamber, electrostatic stainless steel collecting cylinder and different nozzle size (4.0, 5.5 and 7.0 μm) to control particle size during drying process. The samples in the nanospray dryer are sprayed by vibration of piezoelectric driven actuator in the nozzle, then sprayed solution dry in the drying chamber and the produced nanoparticles form as a layer on the wall of collecting cylinder as a result of repulsion of charges presented in nano-spray dryer’s electrode [1].
The nano-spray dryer was used to study the effect of different apparatus conditions on the activity β-galactosidase as a protein model and trehalose as stabilizer. The activity of the prepared β-galactosidase powder was not affected, when the nano-spray dryer was adjusted to 80ºC as inlet temperature using nozzle size; 40 μm and 0% ethanol for the prepared spray solution [2]. Nicergoline nanoparticles were prepared by nano-spray dryer using water: ethanol solution to obtain pure nicergoline nanoparticles with amorphous form to enhance drug solubility. The amorphous form of the drug with particle size of 0.79 μm revealed an increase in drug release and good stability over one year of storage at 3ºC [3].
Inhaled powder of ketoprofen lysine was prepared by nanospray dryer in presence of leucine as dispersity-enhancer using 30% isopropyl alcohol solution, using different nozzle size. High yield value, low process time and suitable powder for inhalation was obtained when 4 μm nozzle was covered with thin layer of span 80 to enhance powder production [4]. Cyclosporine and dexamethasone were dissolved with different weight ratios with PLGA in organic solvent composed from dichloromethane and ethanol (70/30%) and were sprayed through the nano-spray dryer. The produced nanoparticles revealed spherical particles surface with particle size values between 0.90 and 2.23 μm and process yield values varied between 20.05 and 55.5 % [5].
Econazole nitrate was used as a model of antifungal drug in this study. Econazole nitrate is classified as antifungal drug for the treatment of superficial infections such as dermatomycoses and vaginal candidosis [6]. The main disadvantage of econazole nitrate is its water insolubility which considered a challenge in formulation process. To enhance the solubility of econazole nitrate we used hydroxypropyl-β-cyclodextrin (HP-β-CD) as a carrier which responsible for engulfing econazole in the inside lipophlic part by complexation while the outside part of cyclodextrin is hydrophilic.
In our previous study, econazole nitrate nanosuspension was prepared using hydroxypropyl-β-cyclodextrin and methyl-β- cyclodextrin as a carrier, and polyethylene oxide, polyvinylpovidone K 30, Tween 80, poloxamers 407 and Cremophor EL as a stabilizer with using of ethanol as organic solvent. The use of hydroxypropyl- β-cyclodextrin with Tween 80 achieved good particles stability and improving drug release from the prepared nanosuspension [7]. Therefore, in this work, we aimed to study the effect of using different weight ratios of drug to cyclodextrin on the stability and drug release of prepared nanoparticles. In this study, econazole nitrate nanosuspension was formulated using 1:1, 1:2 and 1:3 weight ratios of drug to hydroxypropyl-β-cyclodextrin with 10% Tween 80 using nano-spray dryer. The resulting nanoparticles from nano-spray dryer. The obtained nanoparticles were characterized for their morphology, process yield, percentage drug loading, in-vitro drug release, and particle size and zeta potential stability over 6 months of storage at room temperature.
Materials and Methods
Materials
Econazole nitrate was obtained as a gift from Minapharm Pharmaceuticals, Egypt. Dialysis membrane (cut-off 14000 Da) was purchased from Sigma Aldrich, USA. Hydroxypropyl-betacyclodextrin (HP-β-CD) was kindly donated by Roquette, France. Tween 80 was obtained from Cisme Chemicals, Italy. Sodium chloride, sodium bicarbonate, potassium chloride, calcium chloride dehydrate and absolute ethanol were purchased from El-Nasr Pharmaceutical Chemicals, Egypt. All other reagents were of analytical grade and used as received. All water used was deionized, bi-distilled water.
Methods
Preparation of econazole nitrate nanoparticles: The formulations were prepared by nano-spray dryer using the closed system. Precisely, drug, HP-β-CD and Tween 80 were dissolved in ethanol as presented in Table 1 and the prepared samples were sonicated well for 15 min (Elmasonic S30H, Germany) to ensure completely clarity of the solution, followed by spraying through the Büchi® nano-spray dryer B-90 (Büchi Labortechnik, Switzerland). The prepared samples were sprayed through nozzle size of 7.0 μm using 95ºC for inlet temperature and 45ºC for outlet temperature using gas flow rate of 115 L/m.
Evaluation of econazole nitrate nanoparticles
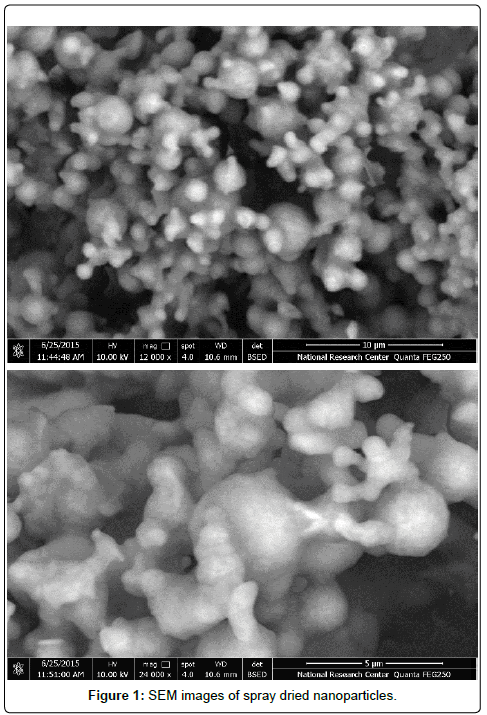
Scanning Electron Microscopy (SEM): The produced nanoparticles were coated with gold and examined at 10kV using scanning electron microscope (Quanta FEG250, USA). The SEM images were taken to determine the shape of nanoparticles produced by nano-spray drying technique.
Determination of nano-spray drying process yield: The nanoparticles produced by nano-spray dryer were calculated for their yield values by the following equation:
% Yield= (Recovered mass / Mass entered in the spray dryer) ×100
Percentage drug loading: The econazole formulations were weighed and then dissolved in ethanol to determine the amount of drug in nanoparticles using Shimadzu 1800 UV (Japan) measured at 271 nm. The percentage drug loading was calculated using the following equation:
% Drug loading = (Amount of drug in nanoparticles / Weight of nanoparticles) ×100
Stability of econazole nitrate nanosuspension: The stability study was performed as prescribed before at Maged et al. [7]. The produced nanoparticles were dispersed in isotonic buffer pH 7.4, and then, they were stored at room temperature for 6 months. The particle size, polydispersity index and zeta potential for the nanoparticles were measured before and after storage using Malvern Nano Zetasizer ZS (U.K).
In-vitro drug release studies: The obtained nanoparticles by nano-spray dryer were dispersed in isotonic phosphate buffer pH 7.4 and were tested by dialysis method for drug release. Econazole loaded nanoparticles were loaded in dialysis cellulose bag with 1 ml isotonic buffer and were closed by two clamps at each end. The cellulose bag was steeped in 100 ml of a simulated tear fluid (0.68 g NaCl, 0.22 g NaHCO3, 0.008 g CaCl2, 0.14 g KCl and distilled deionized water to 100 mL) with 10% ethanol placed in well closed vessel to avoid medium evaporation using shaking rate of 250 stroke per min and maintained at 34ºC (incubator shaker IKA KS 4000, Germany). Samples were withdrawn at the following time intervals 0.5, 1, 2, 3, 4, 5 and 24 hrs. and were measured spectrophotometrically at 221.5 nm.
Cumulative percentage of drug released at 5 hr (Q5hr) and cumulative percentage of drug released at 24 hr (Q24hr) were chosen to compare between the releases patterns for the tested formulations.
The kinetics of econazole nitrate release from nanoparticles was determined by applying Korsmeyer-Peppas equation [8,9]:
Qt / Q∞ = ktn
Where, Qt/Q ∞ is the fraction of drug released at time t, n is the release exponent and indicative of the mechanism of drug release. K is a constant incorporating structural and geometric characteristic of the controlled release device (having units of t-n).
Results and Discussion
Evaluation of econazole nitrate nanoparticles
Scanning Electron Microscopy (SEM): The cyclodextrin nanoparticles showed spherical-like shape with formation of protrusions on the surface of nanoparticles (Figure 1). It can be explained that the drying process caused evaporation of ethanol from the sprayed droplets, leaving the collected nanoparticles as granules, while the ultrasonic vibration of actuator at the nozzle, resulting in formation of some protrusions on the surface of each nanoparticles [7].
Determination of nano-spray dryer process yield: One of the disadvantages with the traditional spray dryer is the low process yield [10]. In our study, the formulated nanoparticles should high process yield values compared to traditional spray dryer, where the yield values were ranging between 79.1 and 80.3 %.
Using HP-β-CD with prepared econazole nitrate nanoparticles in weight ratio of 1:1 drug to CD using 10% Tween 80 (SNP1) revealed no significant difference in its process yield value compared to that prepared with 1:2 weight ratio of drug to HP-β-CD using 10% Tween 80 (SNP2) (p>0.05). On the other hand, increasing the weight ratio of drug to HP-β-CD from 1:2 to 1:3 with the use of 10% Tween 80 (SNP3) showed increase in process yield value (84.9%) compared to that for SNP1 (79.1%) and SNP2 (80.3%) formulations (Table 1). The high process yield values of the resulting nanoparticles can be explained by the low adhesion force for the obtained nanoparticles with the collecting cylinder when cyclodextrin was used as a cryoprotective agent and increasing weight ratio of drug to cyclodextrin, resulting in increasing in process yield value.
| Formulation code | Formulation composition: 10% Tween 80 |
Characterization | |||
|---|---|---|---|---|---|
| ECO:HPβCD weight ratio |
Yield (%)* | Drug loading (%)* | Q5hr | Q24hr | |
| Pure drug | - | - | - | 8.7± 3.5 | 11.2 ± 6.6 |
| SNP1 | 1:1 | 79.1 ± 1.23 | 51.3 ± 1.38 | 54.2 ± 1.2 | 79.1± 1.1 |
| SNP2 | 1:2 | 80.3 ± 0.79 | 51.4 ± 3.78 | 61.2 ± 1.9 | 89.8 ± 2.1 |
| SNP3 | 1:3 | 84.9 ± 2.22 | 50.1 ± 2.01 | 53.4 ± 1.2 | 91.5 ± 0.3 |
Note: * Each value represents the mean ± SD (n=3); ECO: econazole nitrate; HP-β-CD: hydroxylpropyl-beta-cyclodextrin; SNP: spray dried nanoparticles; Q5hr: cumulative percentage drug release after 5 hrs; Q24hr: cumulative percentage drug release after 24 hrs
Table 1: Composition and characterization of prepared econazole nitrate nanoparticles.
Percentage drug loading: All the prepared econazole nitrate formulations in Table 1 revealed high drug loading values equivalent to about 50%. Such high drug loading in the resulted nanoparticles is reflect the importance of nano-spray drying technique for producing nanoparticles with maximum drug loading, and decreasing the amount of nanoparticles needed to be administered in order to give a therapeutic effect.
In-vitro drug release studies
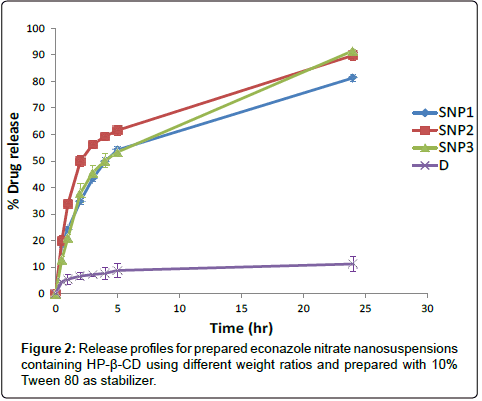
The nanoparticles prepared by nano-spray dryer showed about 6 and 4 times increase in percent drug release after 5 and 24 hours, respectively compared to drug alone (D) that dispersed in isotonic buffer without any additives (p<0.05). This can be explained on the basis that econazole nitrate was presented in amorphous form in the prepared nanoparticles, while crude drug was presented in crystalline form, resulting in decrease of drug release [11].
Increasing weight ratio of drug to HP-β-CD from 1:1 (SNP1) to 1:2 (SNP2) drug to CD with the prepared nanoparticles containing 10% Tween 80 showed significant increase in percent drug released after 5 and 24 hrs (p<0.05) as observed in Figure 2. This could be due to the entrapment of econazole nitrate into the hydrophobic cavity of HP-β-CD that caused an increase in drug dissolution into dissolution medium [12]. Further increase in the weight ratio of drug to HP-β-CD from 1:2 (SNP2) to 1:3 (SNP3) resulted in decrease in percentage drug release after 5 hrs. Such decrease in drug dissolution with increasing the content of HP-β-CD in the formulations can be in response to the instability in the SNP3 formulation and formation of drug crystals as mentioned in the stability study.
The release kinetics of SNP1, SNP2 and SNP3 formulation, according to Korsmeyer-Peppas equation, showed non-fickian transport.
Stability of econazole nitrate nanosuspensions
Nanoparticles have large surface area leads to high total surface energy, which is thermodynamically unstable. Such thermodynamic instability leads to agglomerate of the nanoparticles to reduce the surface energy. Particle agglomeration causes a lot of problems such as crystal growth, rapid settling and Ostwald ripening [13]. Therefore, we did subject our formulations to stability study at room temperature for 6 months.
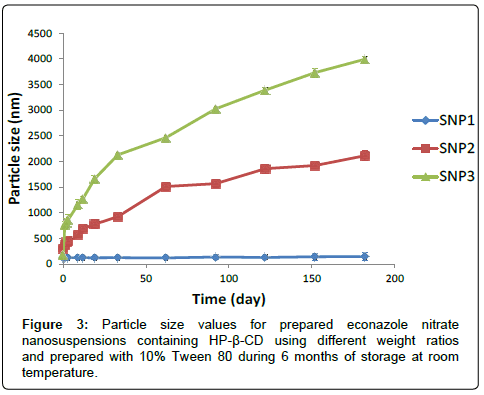
Evaluation of particle size: Figure 3 illustrates the particle size and polydispersity index values for econazole nitrate nanoparticles containing 1:1, 1:2 and 1:3 weight ratio of drug to HP-β-CD and prepared with 10% Tween 80 (SNP1, SNP2 and SNP3, respectively).
The particle size values for the freshly dispersed formulations SNP1, SNP2 and SNP3 were 325.1, 294.1 and 184.7 nm, respectively. Such decrease in nanoparticles size values with increasing cyclodextrin concentration in the nanoparticles could be explained on the basis that increase HP-β-CD concentration could resulting in an increase in amorphous form of the drug, resulting in smaller particle size [14].
The formulation containing 1:1 weight ratio of drug to HP-β-CD (SNP1) revealed stability in particle size values between 325 and 351 nm during the stability period. On the other hand, SNP2 formulation which contains 1:2 weight ratio of drug to HP-β-CD retained its particle size values between 300 and 440 nm during the first 3 days of stability study, and after that it revealed abrupt increase in its particle size values which reached 1511 nm after 2 months of storage at room temperature with PDI value of 1 and its value reached to 2112 nm at the end of the study period. On the other hand, SNP3 formulation which contains 1:3 weight ratio of drug to HP-β-CD showed sudden increase in its particle size value which reached 767 nm after the first day of storage and its value reached to 3992.3 nm after 6 months of storage at room temperature.
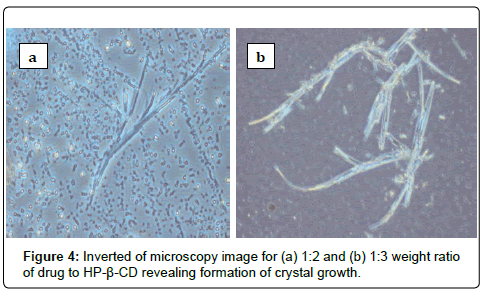
SNP2 and SNP3 formulations showed instability in particle size and PDI values during six months of storage at room temperature compared to those for SNP1 formulation (p<0.05). Increasing weight ratio of drug to HP-β-CD to 1:2 or 1:3, drug to CD, caused a decrease in the stability of drug-CD complex. Such decrease in HP-β-CD complex stability occurred by deprotonisation of hydroxyl groups in the hydrophilic part and the rim of the cyclodextrin cavity with the adjacent hydroxyl groups of another cyclodextrin, resulting in decreasing solubility of drug-CD complex and formation of white large aggregations [15,16]. The inverted microscopy detected formation of crystal growth for both SNP2 and SNP3 formulation as shown in Figure 4 after one month of storage at room temperature.
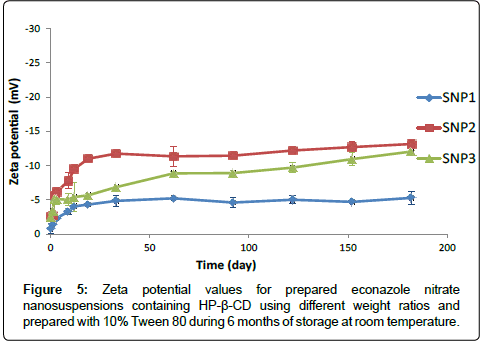
Evaluation of zeta potential: Figure 5 describes the zeta potential values for econazole nitrate nanoparticles containing 1:1, 1:2 and 1:3 weight ratios of drug to HP-β-CD and prepared with 10% Tween 80 (SNP1, SNP2 and SNP3, respectively).
The analysis for the freshly dispersed formulations SNP1, SNP2 and SNP3 revealed zeta potential values; -0.7, -2.5 and -2.3 mV, respectively. Such increase in the negative charge with increasing the content of HP-β-CD in the formulations may be attributed to the large number of oxygen atoms available in the HP-β-CD that have a larger electron cloud, due to the unshared lone pairs, giving negative charge for the formulation.
SNP2 and SNP3 formulations containing 1:2 and 1:3 weight ratio of drug to HP-β-CD, respectively showed increase in their zeta potential values during the stability study and they reached to -13.1 mV for SNP2 formulation and -12.05 mV for SNP3 formulation after the storage period compared to that prepared using 1:1 weight ratio of drug to HP-β-CD (SNP1) (p<0.05) as shown in Figure 4. This instability was explained previously in the section of particle size stability study.
Conclusion
The cyclodextrin nanoparticles that formulated by nano-spray dryer revealed high yield value and percent drug loading. The prepared nanoparticles had particle size values ranging between 184.7 and 325.1 nm. Increasing weight ratio of drug to cyclodextrin from 1:1 to 1:2 and 1:3 revealed increase in percent drug release after 24 hr. On other hand, increasing weight ratio of cyclodextrin affected the physical stability of the particles in the nanosuspension with formation of crystal growth after study period.
References
- Arpagaus C (2011) Nano Spray Dryer B-90: Literature review and applications. BÜCHI Labortechnik AG.
- Bürki K, Jeon I, Arpagaus C, Betz G (2011) New insights into respirable protein powder preparation using a nano spray dryer. Int J Pharm 408: 248-256.
- Martena V, Censi R, Hoti E, Malaj L, Martino PD (2012) A new nanospray drying method for the preparation of nicergoline pure nanoparticles. J Nano Res 14: 934.
- Aquino RP, Stigliani M, Del Gaudio P, Mencherini T, Sansone F, et al. (2014) Nanospray drying as a novel technique for the manufacturing of inhalable NSAID powders. ScientificWorldJournal 2014: 838410.
- Schafroth N, Arpagaus C, Jadhav UY, Makne S, Douroumis D (2012) Nano and microparticle engineering of water insoluble drugs using a novel spray-drying process. Colloids Surf B Biointerfaces 90: 8-15.
- Heel RC, Brogden RN, Speight TM, Avery GS (1978) Econazole: a review of its antifungal activity and therapeutic efficacy. Drugs16: 177-201.
- Maged A, Mahmoud AA, Ghorab MM (2016) Ghorab, Nano-spray drying technique as a novel approach to formulate stable econazole nitrate nanosuspension formulations for ocular use. Mol Pharm 13: 2951-2965.
- Korsmeyer RW (1983) Mechanisms of solute release from porous hydrophilic polymers. International J Pharmaceutics 15: 25-35.
- Peppas NA (1985) Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv60: 110-111.
- Arpagaus C (2012) A novel laboratory-scale spray dryer to produce nanoparticles. Drying Technology 30: 1113-1121.
- Jog R, Burgess DJ (2016) Pharmaceutical Amorphous Nanoparticles. J Pharm Sci106: 39-65.
- Kim JE, Cho HJ, Kim DD (2014) Budesonide/cyclodextrin complex-loaded lyophilized microparticles for intranasal application. Drug Dev Ind Pharm 40: 743-748.
- Lindfors L, Skantze P, Skantze U, Rasmusson M , Zackrisson A, Olsson U, et al. (2006 ) Amorphous drug nanosuspensions. 1. Inhibition of Ostwald ripening. Langmuir22: 906-910.
- Choi HG, Kim DD, Jun HW, Yoo BK, Yong CS (2003) Improvement of dissolution and bioavailability of nitrendipine by inclusion in hydroxypropyl-β-cyclodextrin. Drug Dev Ind Pharm 29: 1085-1094.
- Loftsson T, Jarho P, Másson M, Järvinen T (2005) Cyclodextrins in drug delivery. Expert Opin Drug Deliv 2: 335-351.
- Jarho P, Vander Velde D, Stella VJ (2000) Cyclodextrinâ€Âcatalyzed deacetylation of spironolactone is pH and cyclodextrin dependent. J Pharm Sci 89: 241-249.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi