Research Article, J Pharm Sci Emerg Drugs Vol: 6 Issue: 1
Method Validation for Equilibrium Solubility and Determination of Temperature Effect on the Ionization Constant and Intrinsic Solubility of Drugs
Kyoowon Baek1†, Su Bin Jeon2†, Bo Kyung Kim2 and Nam Sook Kang2*
1Sirius Analytical, RN 602-2nd, Daeun Plaza, 818-5, Yangchungri, Ochangup, Cheongwonkun, CHungbuk, Korea
2GraduateSchool of New Drug Discovery and Development, Chungnam National University, Korea
†These authors contributed equally to this work
*Corresponding Author : Nam Sook Kang
School of New Drug Discovery and Development, Chungnam National University, Daehakno 99, Yuseong-gu, Daejeon 305-764, Korea
Tel: +82-010-7292-5756
E-mail: nskang@cnu.ac.kr
Received: January 01, 2018 Accepted: February 20, 2018 Published: February 26, 2018
Citation: Baek K, Jeon SB, Kim BK, Kang NS (2018) Method Validation for Equilibrium Solubility and Determination of Temperature Effect on the Ionization Constant and Intrinsic Solubility of Drugs. J Pharm Sci Emerg Drugs 6:1. doi:10.4172/2380-9477.1000125
Abstract
We performed this study to understand the physicochemical properties of drug-like molecules such as pKa and logS0 (logS) values. The first purpose is to compare pKa and logS0 values obtained using a prediction tool and experimental results. The second purpose is to identify the temperature effect of pKa and logS0 for ionizable drugs at 25ºC and 37ºC. PKa and logS0 of ordinary base compounds were significantly changed by increasing the temperature, but acid and amphoteric compounds were not. The third purpose is to validate the shake-flask method using different buffers and stirring and sedimentation times compared with potentiometric experiments. The equilibrium solubility from the shake-flask method had an excellent consistency with the potentiometric method (CheqSol) for crystalline forms. Additionally, measurement of amorphous compounds, which are poorly soluble and metastable in aqueous solutions, was feasible to determine logS0 (and logS) values using the CheqSol technique.
Keywords: Drug-like molecules; Ionization constant; Equilibrium solubility; CheqSol
Introduction
Approximately 95% of marketed drugs are ionizable in aqueous or any organic solvents. Once they are ionized, the ionization constant (pKa) is considered to be the predominant parameter in drug-like molecule solutions. It also influences lipophilicity and solubility (logS0 for intrinsic and logS for equilibrium), which are required to estimate physiological drug absorption and distribution. These active pharmaceutical ingredient (API) terms are the most important physicochemical properties for the pre formulation of drugs.
There are many tools available to estimate physicochemical properties, especially pKa and logS0 values, instead of measuring using specific techniques. Of the many techniques used to obtain reliable values, tools such as MarvinSketch (ChemAxon Ltd.) [1], ADMET Predictor (Simulation Plus Inc.) [2], and ACD/Percepta (ACD/Labs Build 2203, Toronto, Canada) [3] are frequently used to predict pKa and logS0. These described tools were recently developed and are widely used in pharmaceutical fields. In addition, two-dimensional (2D)- and three-dimensional (3D)-quantitative structure-property relationships (QSARs) have also been used to model pKa values for many years [4]. Predicting the pKa and logS0 is easy and convenient by identifying the structure of drug-like molecules based on their 2D structure. However, it was reported that the prediction accuracy is somewhat dependent on the mechanisms of individual software and on the characterizations of drug-like molecules [3,4]. Therefore, the first purpose of this study is to compare pKa and logS0 values from the experiments with the predicted values obtained using ACD/ Percepta software.
Theoretical physicochemical properties, especially the thermodynamic aspect of pKa for ionizable compounds, are dependent on the temperature. In addition, physiological absorption and distribution of APIs are necessarily required to estimate in bio relevant temperature (37ºC). However, most of the published physicochemical properties including pKa and logS0 were primarily determined under standard conditions, which means at room temperature of 25ºC and at a 0.15M ionic strength. Few studies have reported the effect at biorelevant temperatures [5-7]. Therefore, it is necessary to investigate whether the bio relevant temperature of 37ºC has an effect on the pKa and logS0 of ionizable drugs compared with room temperature of 25ºC using an automated potentiometric titration method.
Solubility is theoretically categorized by three definitions: kinetic solubility, meaning the concentration of a compound when a precipitation first appears in the solution; equilibrium solubility (logS), meaning the concentration of a compound in a saturated solution when excess solid is present and the solution and solid are at equilibrium (pH-dependent); and intrinsic solubility (logS0), referring to the equilibrium solubility of the free acid and base form at a pH where it is fully unionized and thus pH-independent [8,9]. Many different methods have been reliably developed to measure solubility. The classical shake-flask method is still considered to be the basic technique and it has been widely used for a long time. The shake-flask method is a simple procedure, but it is time consuming to manually shake and equilibrate the samples [9]. It represents the equilibrium solubility, which is pH dependent, so that many points at a different pH are normally prepared to interpret solubility profiles over a pH range. In this study, the chasing equilibrium solubility (CheqSol) method, which has been an approved technique for many years [9-11], was used to measure the intrinsic solubility and compare the equilibrium solubility at a designated pH, which was measured using the shake-flask method.
Materials and Methods
Chemicals and materials
All test compounds were purchased from Sigma (Sigma-Aldrich, Korea), including small crystals or powders with a high purity (≥ 98%). These were used without additional purifications for this study. HPLC-grade water was purchased from Fisher (Thermo Fisher, Korea) and organic solvents such as methanol and dimethyl sulfoxide DMSO (Merck, Korea) were also supplied individually. Monobasic potassium phosphate (99%), 0.5M hydrochloric acid, and 0.5M potassium hydroxide were all purchased from the Samchun Chemical Co. Inc. (Korea) and disodium hydrogen phosphate from the Kanto Chemical Co. Inc. (Japan).
Active pharmaceutical ingredients
A total of 15 drug-like molecules, including six acids, seven bases, and two amphoteric compounds were chosen for this study. Because the shake-flask solubility experiments required the use of a micro plate spectrophotometer, they are all chromophore, which are active in UV absorbance and are poorly soluble in the aqueous environment. Table 1 summarizes the identification of all compounds, including predicted pKa values. Some acid compounds such as diclofenac, diflunisal, furosemide, ibuprofen, and ketoprofen contain carboxylic acid group (COOH-) and the others are ordinary base and amphoteric compounds. Common water mixable organic solvents such as MeOH and DMSO were inevitably used to measure pKa of poorly insoluble compounds in aqueous solution. The use of cosolvents causes shifts in the titration curve and derives the psKa in the presence of cosolvent, not aqueous pKa. Sequentially, the aqueous pKa values were calculated using the Yasuda-Shedlovsky extrapolation method, which represents linear regression with a function of multiple psKa versus wt% of cosolvents [3-5].
| Compounds | Class | Ionizable Group | Predicted pKa | Experimental pKa | ΔpKa1) | ΔpKa2) | |
|---|---|---|---|---|---|---|---|
| @25ºC | @ 37ºC | ||||||
| Albendazole | Base | Imidazole | 5.37 | 4.15 | 4.02 | 1.22 | 0.13 |
| Acid | Imidazole | 11.0 | 10.4 | 10.2 | 0.60 | 0.20 | |
| Chlorpheniramine | Base | pyridine | 4.46 | 3.82 | 3.40 | 0.64 | 0.42 |
| Base | amine | 9.33 | 9.29 | 8.95 | 0.04 | 0.34 | |
| Diclofenac | Acid | carboxylic | 4.18 | 4.02 | 4.05 | 0.16 | 0.03 |
| Diflunisal | Acid | carboxylic | 2.94 | 2.67 | 2.71 | 0.27 | 0.04 |
| Furosemide | Acid | carboxylic | 3.04 | 3.75 | 3.54 | 0.71 | 0.21 |
| Acid | thiadiazine | 9.79 | 10.4 | 10.5 | 0.61 | 0.10 | |
| Haloperidol | Base | piperidine | 8.04 | 8.92 | 8.42 | 0.88 | 0.50 |
| Hydrochlorothiazide | Acid | thiadiazine | 8.95 | 8.72 | 8.60 | 0.23 | 0.12 |
| Acid | sulfonamide | 9.57 | 9.97 | 9.93 | 0.40 | 0.04 | |
| Ibuprofen | Acid | carboxylic | 4.41 | 4.37 | 4.30 | 0.04 | 0.07 |
| Imipramine | Base | amine | 9.49 | 9.68 | 9.06 | 0.19 | 0.62 |
| Ketoconazole | Base | piperazine | 3.58 | 4.17 | 3.42 | 0.59 | 0.75 |
| Base | Imidazole | 6.88 | 6.59 | 6.05 | 0.29 | 0.54 | |
| Ketoprofen | Acid | carboxylic | 4.20 | 3.99 | 4.11 | 0.21 | 0.12 |
| Papavarine | Base | pyridine | 6.32 | 6.50 | 6.25 | 0.18 | 0.25 |
| Piroxicam | Base | pyridine | 3.53 | 1.88 | 1.91 | 1.65 | 0.03 |
| Acid | hydroxyl | 4.50 | 5.30 | 5.29 | 0.80 | 0.01 | |
| Propranolol | Base | amine | 9.50 | 9.56 | 9.10 | 0.06 | 0.46 |
| Verapamil | Base | amine | 9.00 | 8.97 | 8.60 | 0.03 | 0.37 |
2ΔpKa = Experimental pKa @25亷− Experimental pKa @37亷
Table 1: Comparison of predicted and experimental pKa values.
Automated potentiometric titration
SiriusT3 (Sirius Analytical Ltd.) was used to determine pKa and logS0 (logS) for all APIs at 25 ± 0.1ºC and 37 ± 0.1ºC. It is an automated titration system with functions of pH electrode (pH-metric) and UV/VIS spectrophotometer (UV-metric) in the range of pH 2 to 12. The instrument uses 0.15M KCL water, 0.5M HCl, 0.5M KOH, and 80% MeOH (60% DMSO). All specific titrants or solvents for individual purposed assays are automatically transferred into sample vials and the instrument performs pH-metric (or UV-metric) titration by changing pH in the unit by 0.2 from low to high pH or from high to low pH, based on the compound type.
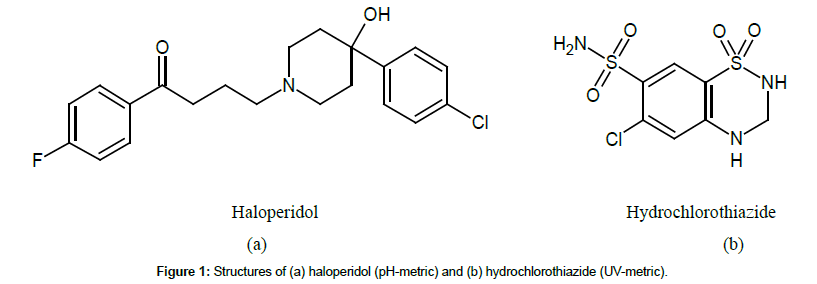
For pKa measurement, pH-metric and UV-metric titrations were selectively applied according to the location of ionizable groups. Figure 1 show the structures of haloperidol and hydrochlorothiazide, which were tested for pH-metric and UV-metric pKa titration, respectively. As shown in hydrochlorothiazide structure, all ionizable groups are located at or close to a chromophore, which is able to measure the UV-metric titration. pKa measurements using UV-metric assay are quick and reproducible for compounds such as hydrochlorothiazide [9]. Haloperidol also contains a chromophore but an ionizable group (piperidine) is located at a distant point from both chromophore sites. This compound is not active enough on UV, so has to test in pH-metric pKa titration [3-8].
Shake-flask method for solubility
Edit et al. [9] tested logS0 using the shake-flask method with different buffer solutions and various stirring and sedimentation times. Based on their conclusions about estimating excellent results, using Sörensen I buffer solution with 1/15M Na2HPO4 and 1/15M KH2PO4 prepared at 0.076M ionic strength was used in this study. This phosphate buffer was approximately pH 4.0 to 9.0 and the designated pH was validated using a benchtop pH meter (Suntex, Taiwan) using 0.5M HCl (or 0.5M KOH) at the range of pH<4 and pH>9. After adding excess amounts of compounds, the saturated solution was sustained by stirring for 6 hours and allowing sedimentation for 18 hours in a water bath (Daihan Science, Korea) at 25ºC. Before analysis using a microplate spectrophotometer (EPOCH, BioTek Instruments Inc.), the samples were collected using a micro pipette followed by phase-separation and filtration. All stock solutions at various concentration ranges for individual compounds were applied to UV spectrophotometer for the quantitation analysis and all 15 calibration curves were determined using a function of the concentration and the absorbance, which was represented by r2>0.99 for all compounds, except for haloperidol (r2=0.92).
Results and Discussion
pKa and logS0 values of prediction vs. experiment at 25°C
Even though there are many commercial tools available to estimate physicochemical properties of APIs, ACD/Percepta (ACD/ Labs) based on the 2D structure is popular in chemistry labs, and it was used in this study to estimate pKa and logS0 values. As summarized in Tables 1 and 2, all predicted values of pKa and logS0 values were obtained at room temperature (25°C), not at a bio relevant temperature (37ºC). Therefore, predicted values for pKa and logS0 could be compared only with experimental values at 25ºC.
| Compounds | Class | Predicted logS0 | Experimental logS0 | ΔlogS01) | ΔlogS02) | |
|---|---|---|---|---|---|---|
| @25ºC | @37ºC | |||||
| Albendazole | Ampholyte | -4.51 | ND3) | ND | - | - |
| Chlorpheniramine | Base | -3.10 | -2.66 | -2.38 | 0.4 | 0.3 |
| Diclofenac | Acid | -4.51 | -5.44 | -5.21 | 0.9 | 0.2 |
| Diflunisal | Acid | -4.21 | -4.60 | -4.84 | 0.3 | 0.2 |
| Furosemide | Acid | -4.37 | -4.05 | -4.07 | 0.3 | 0.0 |
| Haloperidol | Base | -3.93 | -5.14 | -4.70 | 1.2 | 0.4 |
| Hydrochlorothiazide | Acid | -2.23 | -2.74 | -2.51 | 0.5 | 0.2 |
| Ibuprofen | Acid | -3.08 | -4.19 | -3.98 | 1.1 | 0.2 |
| Imipramine | Base | -4.60 | -4.28 | -3.88 | 0.3 | 0.4 |
| Ketoconazole | Base | -3.92 | -4.03 | -3.68 | 0.1 | 0.4 |
| Ketoprofen | Acid | -3.37 | -3.24 | -3.06 | 0.1 | 0.2 |
| Papavarine | Base | -4.28 | -4.25 | -3.55 | 0.0 | 0.7 |
| Piroxicam | Ampholyte | -2.92 | -4.68 | -4.48 | 1.8 | 0.2 |
| Propranolol | Base | -2.81 | -3.51 | -2.55 | 0.7 | 1.0 |
| Verapamil | Base | -4.57 | -4.20 | -3.90 | 0.4 | 0.3 |
2ΔlogS0 = Experimental logS0 @25亷− Experimental logS0 @37亷
3ND = not determined
Table 2: Summary of predicted and experimental logS0 values at 25ºC and 37ºC.
The comparison between predicted and experimental pKa values was the same, as indicated by a ΔpKa of less than ± 0.3 pH units. However, the predicted pKa values of several compounds such as albendazole, chlorpheniramine, furosemide, haloperidol, ketoconazole, and piroxicam were different compared with the measured pKa values in various functional groups; these ΔpKa values ranged from ±0.4 to ±1.65 pH units. Francesca et al. [4] reported similar results based on the corresponding ionizable groups using their new model. Additionally, Fan et al. [8] indicated a significant pKa difference (> ± 0.4 pH units) of N-isoleucyl-4-methyl-1,1- cyclopropyl-1-(4-chlorine)phenyl-2-amylamine HCl (JFD), a novel investigational anti-obesity drug without obvious cardiotoxicity, by comparing the predicted values using ACD/Labs software and the experimental values.
Table 2 summarizes the difference in the predicted and the experimental logS0 values. Unlike the comparison of pKa values, most compounds except for diflunisal, ketoconazole, ketoprofen, and papavarine showed that ΔlogS0 was greater than ±0.3 log solubility units. Few studies have reported predicting logS0 values of APIs using various prediction models, because solubility is difficult to predict as a result of several factors such as solute and solvent purity, precipitation behaviour, and stability in solution [12].
Temperature effect for pKa and logS0 at 25ºC and 37ºC
To evaluate the temperature effect for pKa and logS0 values, all APIs were titrated under the assigned temperatures, which is room temperature and the bio relevant temperature. Table 2 indicates individual pKa assay results at different temperatures, which were validated using pKa calculation software (SiriusT3 Refinement Software, Sirius Analytical Ltd.).
Na Sun et al. [5] identified the temperature effect of pKa values using 143 APIs and reported pKa values of some overlapped compounds measured in this study such as furosemide, haloperidol, hydrochlorothiazide, imipramine, papavarine, piroxicam, propranolol, and verapamil. All pKa values of these compounds reported by Na Sun et al. [5] are consistent with the results of this study. As shown in Table 2, different temperatures did not affect ampholyte and acid compounds. However, base compounds were significantly influenced by the biorelevant temperature for chlorpheniramine, haloperidol, imipramine, ketoconazole, papavarine, propranolol, and verapamil. Thus, compounds that contain simple carboxylic and hydroxyl groups have almost the same pKa at 25ºC and 37ºC. However, pKa values of all simple base groups including imidazole, amine, and pyridine were decreased by an increasing temperature. Richard et al. [6] also reported a decrease in pKa of fentanyl at 25ºC, which is a base compound containing a piperidine group at the ionizable site, compared with 37ºC, and this decrease was by greater than ± 0.3 pH units.
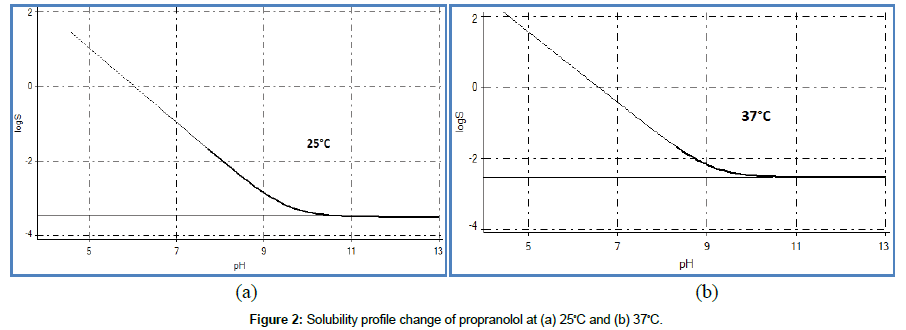
According to the logS0 values at different temperatures, most compounds are theoretically expected to be more soluble at a higher temperature and solubility of salts such as maleate, sodium, and chloride are expected to increase [9]. However, these effects were not recognized in this study. Table 2 shows that the intrinsic solubility of all base compounds was meaningfully increased by more than ± 0.3 log solubility units at the biorelevant temperature compared with room temperature. This result could be related to pKa changes at different temperatures, as suggested above. pKa values of all base compounds were significantly influenced by increasing temperatures, which suggests that the solubility was increased at the biorelevant temperature. However, the temperature had no effect on all acid and amphoteric compounds, as shown in Table 2. Similar to pKa variations in temperature effects, these results could be interpreted using the functional groups contained on each compound, but further studies with other drug-like molecules may be necessary. Figure 2 indicates the solubility profile as a function of pH for propranolol. In this case, the effect of temperature caused an increase in logS0 from −3.51 (± 0.04) to −2.55 ( ± 0.01) at pH 10 to 13. In the range of pH 2 to 10, propranolol was fully ionized and the ion species were not dominant in precipitated solutions. This region represents the equilibrium solubility (logS) from the solubility profile, not the intrinsic solubility (logS0), suggesting that it is dependent on pH.
Equilibrium solubility (logS) between the shake-flask method and the potentiometric method
To measure the solubility concentrations of all APIs, two different techniques, the classical shake-flask method and the potentiometric method (CheqSol), were used in this study. The shake-flask is known to be a basic method to estimate equilibrium solubility. However, this method normally requires several experimental factors to be considered, such as stirring time, sedimentation time, composition of aqueous buffers, temperature, amount of solid excess, and phaseseparation methods [9]. The potentiometric method, however, can be performed using CheqSol, and it requires less-than-milligram amounts of a sample for 1 to 1.5 hours titrations [13]. Both techniques were used in this study at the specific pH, and the equilibrium solubility values were compared. Table 3 shows the logS values obtained using different techniques at a fully unionized pH at 25ºC.
| Compounds | pH | Neutral Species (%) | Shake-Flask logS |
CheqSol logS |
ΔlogS1) |
|---|---|---|---|---|---|
| Albendazole | 7.4 | >99 | -4.69 | ND2) | - |
| Chlorpheniramine | 11.5 | >99 | -2.28 | -2.62 | 0.3 |
| Diclofenac | 2.0 | >99 | <-5.38 | -5.44 | 0.1 |
| Diflunisal | 2.0 | 82.4 | <-4.87 | -4.40 | 0.5 |
| Furosemide | 2.0 | 98.3 | -4.69 | -4.04 | 0.7 |
| Haloperidol | 11.5 | >99 | -4.68 | -4.77 | 0.1 |
| Hydrochlorothiazide | 7.4 | 94.9 | -2.89 | -2.71 | 0.0 |
| Ibuprofen | 2.0 | >99 | ≈-2.99 | -4.19 | 1.2 |
| Imipramine | 11.5 | 98.5 | -4.15 | -4.27 | 0.1 |
| Ketoconazole | 7.4 | 86.6 | <-3.82 | -3.96 | 0.1 |
| Ketoprofen | 2.0 | >99 | -3.82 | -3.30 | 0.5 |
| Papavarine | 7.4 | 88.8 | -4.29 | -4.19 | 0.1 |
| Piroxicam | 7.4 | >99 | -4.79 | -4.75 | 0.0 |
| Propranolol | 11.5 | 98.9 | -3.91 | -3.50 | 0.4 |
| Verapamil | 11.5 | >99 | -4.57 | -4.31 | 0.3 |
2ND = not determined
Table 3: Comparison of logS at unionized pH using shake-flask method and CheqSol.
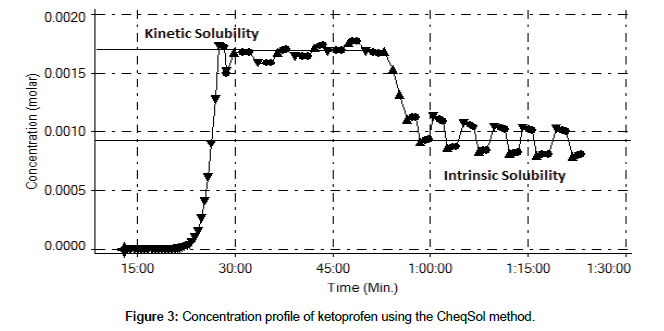
The shake-flask method showed logS values that were compatible with those of the CheqSol method, except for diflunisal, furosemide, ibuprofen, and ketoprofen, which precipitated and existed in their amorphous form in supersaturated solution. Figure 3 shows a concentration profile as a function of time for ketoconazole. Once it was precipitated and had a super saturation status around 30 min, kinetic solubility was observed and remained for 1 hour. Finally, the concentration rapidly decreased to 0.001M, which resulted in intrinsic solubility. The amorphous precipitate is specifically metastable in solution, and it has a strong tendency to convert to a crystalline form [8]. The polymorphic nature of APIs could not be directly detected using the shake-flask method, so the results show significant deviations from the CheqSol results. The solubility of albendazole is experimentally difficult to analyze using the potentiometric method because of its precipitation property. It is a poorly insoluble compound in aqueous solution and it usually exists as the precipitate floating on the aqueous surface.
Edit et al. [9] performed the measurement using the shake-flask method and CheqSol to compare the logS values of hydrochlorothiazide at pH 6.0, which is a crystalline form. They reported that the shakeflask method determined logS to be −2.73 ± 0.01, which is comparable with the logS value of −2.89 at pH 7.4, which is presented in Table 3; no significant deviation is indicated. Therefore, this suggests that the shake-flask method could be validated, especially for crystalline compounds.
Conclusions
The prediction software provided inconsistent values compared with the experimental values especially for logS0, based on comparisons between the predicted and experimental pKa and logS0 values. This suggests that all drug-like molecules should be tested rather than using the prediction tool to understand in vivo drug behaviour. For the temperature effect for pKa and logS0, we showed that pKa and logS0 values only for base compounds were influenced by increasing temperature. The reason that temperature had no effect on acidic and amphoteric compounds requires further study. Additionally, the simple shake-flask method with a microplate spectrophotometer was developed and validated by comparing logS values at a specific pH with the potentiometric method (CheqSol). There was excellent consistency between both methods for crystalline compounds. However, the potentiometric method (CheqSol) is also feasible for all crystalline and amorphous forms. This study was conducted using the shake-flask method only at one pH point to examine the concentration at super saturation. However, multiple points in the pH range might be required in future studies to compare logS0 and logS values with more drug-like molecules, especially amorphous compounds.
Acknowledgments
This study was financially supported by research fund of Chungnam National University.
Author Contributions
All authors performed the study. S.B. Jeon and K.W. Baek performed the experiments, and prepared the figures and manuscript. B.K. Kim prepared the compounds and the other materials. N.S. Kang designed the research, analyzed the data, and critically edited the manuscript for content.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chem Axon (2007) MarvinSketch 4.1.8. Xhem Axon Kft. Budapest, Hungary.
- ADMET Predictor (2014) Simulations Plus, Inc. Lancaster, CA, USA.
- Butcher G, John C, Alex A (2015) pKa-critical interpretations of solubility-pH profiles: PG-300995 and NSC-639829 case studies. ADMET and DMPK 3: 131-140.
- Francesca M, Loriano S, Gianluca S, Gabriele C (2007) New and original pKa prediction method using grid molecular interaction fields. J Chem Inf Model 47: 2172-2181.
- Sun N, Alex A (2011) Biorelevant pKa (37°C) predicted from the 2D structure of the molecule and its pKa at 25°C. J Pharm Biomed Anal 56: 173-182.
- Thurlkill RL, Cross DA, Scholtz JM, Pace CN (2005) pKa of fentanyl varies with temperature: Implications for acid-base management during extremes of body temperature. J Cardiothorac Vasc Anesth 19: 759-762.
- Canari R, Eyal AM (2004) Temperature effect on the extraction of carboxylic acids by amine-based extractants. Ind Eng Chem Res 43: 7608-7617.
- Fan Y, Yang M, Wang Y, Li Y, Zhou Y, et al. (2014) Preformulation characterization and in vivo absorption in beagle dogs of JFD, a novel anti-obesity drug for oral delivery. Drug Dev Lnd Pharm 41: 801-811.
- Baka E, Comer JEA, Takacs-Novak K (2008) Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound. J Pharm Biomed Anal 46: 335-341.
- Karl B, Comer JE, Tom G, Stuart M (2009) New ideas about the solubility of drugs. Chemistry & Biodiversity 6: 1767-1788.
- Ali S, Abolghasem J (2011) Comparison of four models to predict intrinsic solubility of drugs. Lat Am J Pharm 30: 1525-1530.
- David SP, John BOM (2014) Is experimental data quality the limiting factor in predicting the aqueous solubility of druglike molecules? Mol Pharm 11: 2962-2972.
- Vizserálek G, Balogh T, Takács-Novák K, Sinkó B (2013) PAMPA study of the temperature effect on permeability. Eur J Pharm Sci 53: 45-49.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi